Estuarine Health Assessment Using Benthic Macrofauna
As of January 2004 au.riversinfo.org is archived under Precision Info
RIVERSINFO AUSTRALIA ARCHIVE
This archive provides non stylised versions of selected reference documents (formerly) resident on au.riversinfo.org.
Estuarine Health Assessment Using Benthic Macrofauna
John Moverley & Alastair Hirst
Report to: Water Services Association of Australia
From: Museum Victoria
GPO Box 666E Melbourne Vic 3001
Contents
Brief Contents
Foreword & Publication Details
Summary & Acknowledgements
1. Introduction
2. Estuarine Environments
3. General Material And Methods
4. Sample Profile
5. Multivariate Analysis
6. RIVPACS Model Approach
7. Alternative Methods
8. Conclusions and recommendation
9. References
Appendix 1 Abiotic Effects on Univariate Indices
Appendix 2 Two-Way Tables
Appendix 3 Model Predictions
Appendix 4 k-dominance curves
Detailed Contents
Foreword & Publication Details
Summary & Acknowledgements
- Objectives
- Methods
- Findings
- South-eastern Australian Estuarine Communities
- RIVPACS Model
- K-dominance Curves
- Recommendations
1. Introduction
- 1.1 Defining Estuarine Habitat
- 1.2 Estuarine Health Assessment
- 1.3 Sampling Methods
- 1.4 Estuarine Community Characteristics
2. Estuarine Environments
- 2.1 Estuary Mouth
- 2.2 Vertical Mixing
- 2.3 Horizontal Mixing
- 2.4 Estuary Models
3. General Material And Methods
- 3.1 Estuary Selection
- 3.2 Site Selection
- 3.3 Grab samples
- 3.4 Dredge Samples
- 3.5 Sample Processing
- 3.6 Abiotic Variables
- 3.7 Data Manipulation
- 3.8 Temporal Study
4. Sample Profile
- 4.1 Sample Characteristics
- 4.1.1 Results
- 4.1.2 Discussion
- 4.2 Abiotic Effects on Abundance and Diversity
- 4.2.1 Salinity
- 4.2.1.1 Results
- 4.2.1.2 Discussion
- 4.2.2 Vertical Stratification
- 4.2.2.1 Results
- 4.2.2.2 Discussion
- 4.2.3 Seagrasses
- 4.2.3.1 Results
- 4.2.3.2 Discussion
- 4.2.4 Algae
- 4.2.4.1 Results
- 4.2.4.2 Discussion
- 4.3 Estuarine Categories
- 4.3.1 Results
- 4.3.2 Discussion
- 4.4 Classification of sample sites
- 4.5 Species Accumulation Curves
- 4.5.1 Results
- 4.5.2 Discussion
- 4.6 Conclusions
5. Multivariate Analysis
- 5.1 Hierarchical Clustering
- 5.1.1 Methods
- 5.1.2 Results
- 5.1.3 Discussion
- 5.2 Two-way Table
- 5.2.1 Introduction
- 5.2.2 Methods
- 5.2.3 Results
- 5.2.3.1 Grab Samples
- 5.2.3.2 Dredge Samples
- 5.2.4 Discussion
6. RIVPACS Model Approach
- 6.1 Methods
- 6.1.1 Environmental Data
- 6.1.2 Faunal Data
- 6.1.3 Model Construction
- 6.1.4 Test Sites
- 6.1.5 Data Matrix
- 6.2 Results
- 6.2.1 Discriminant Analysis
- 6.2.2 Testing
- 6.2.3 Family Level Identifications
- 6.3 Discussion
7. Alternative Methods
- 7.1 Multivariate Analysis of Abundance Data
- 7.1.1 Methods
- 7.1.2 Results
- 7.1.3 Discussion
- 7.2 K-dominance Curves
- 7.2.1 Methods
- 7.2.2 Results
- 7.2.3 Discussion
- 7.2.4 Testing Improving Estuarine Health, Tasmania
- 7.2.4.1 Results
- 7.2.4.2 Discussion
- 7.2.5 Testing Temporal Patterns – South-eastern Australia
- 7.2.5.1 Methods
- 7.2.5.2 Results
- 7.2.5.3 Discussion
- 7.2.6 Testing Temporal Patterns – Central Queensland
- 7.2.6.1 Methods
- 7.2.6.2 Results
- 7.2.6.3 Discussion
- 7.2.7 Assessment of k-dominance curves
8. Conclusions and recommendation
- 8.1 RIVPACS-type approach
- 8.2 ABC evaluation
- 8.3 Estuarine Health Assessment Protocol
9. References
Appendix 1 Abiotic Effects on Univariate Indices
- A1.1 Geographical Location
- A1.2 Depth
- A1.3 Sediment Organic Content
Appendix 2 Two-Way Tables
- Two-way table of grab sample data
- Two-way table of dredge sample data
Appendix 3 Model Predictions
- Wingan Inlet, lower (EH07)
- Merriman Creek, upper (EH18)
- Lake Tyers, mid (EH29)
- Lake Yambuk, upper (EH53)
- Curalo Lagoon, body (EH72)
- Tamago River, lower (EH81)
Appendix 4 k-dominance curves
- K-dominance curves for dredge samples
- K-dominance curves for grab samples
- Foreword
- Publication Information
Foreword
This report describes the outcomes of a research project conducted under the Urban Research and Development sub-program of the National River Health Program (NRHP).
The NRHP is an on-going national program established in 1993, managed by the Land and Water Resources Research and Development Corporation (LWRRDC) and Environment Australia. Its mission is to improve the management of Australia's rivers and floodplains for their long-term health and ecological sustainability, through the following goals:
- To monitor and assess the health of Australia's rivers.
- To enhance the management of river flows, water allocation and water use to ensure the sustainability of river and floodplain ecosystems.
- To encourage active management to improve the health of Australia's rivers, based on a sound understanding of the ecological and hydrological processes.
- To evaluate the effectiveness of river management actions at a national scale.
Urban streams and estuaries (i.e. those affected by runoff and discharges from urban areas) are an important subset of Australia's waterways. Most are degraded biologically, physically and chemically and therefore require appropriate methods to be developed for health assessment and management. The Urban R&D Sub-program, managed by the Water Services Association of Australia, comprises 8 research projects which were developed to meet research priorities for urban streams and estuaries within the goals of the NRHP and to complement existing NRHP projects on non-urban rivers. Thus, research focuses on development of standardised methods for assessing
the ecological health of urban streams and estuaries which can be linked with data on water and sediment quality. The urban R&D projects commenced in 1996.
The definition of health in urban waterways used is "the ability to support and maintain a balanced, integrative, adaptive community of organisms having a species composition, diversity and functional organisation as comparable as practicable to that of natural habitats of the region".
The eight projects of the Urban Sub-Program are:
| Decision support system for management of urban streams | Dr John Anderson
Southern Cross University, Lismore |
| RIVPACS (River InVertebrate Prediction and Classification System) for urban streams | Dr Peter Breen
CRC for Freshwater Ecology, Monash
University, Melbourne |
| DIPACS (Diatom Prediction and Classification System) for urban streams | Dr Jacob John
Curtin University, Perth |
| Sediment chemistry- macroinvertebrate fauna relationships in urban streams | Dr Nick O'Connor
Water EcoScience, Melbourne |
| Classification of estuaries | Dr Peter Saenger
Southern Cross University,Lismore |
| Literature review of ecological health assessment in estuaries | Mr David Deeley
Murdoch University, Perth |
Estuarine health assessment using benthic macrofauna
| Dr Gary Poore
Museum of Victoria, Melbourne |
| Estuarine eutrophication models | Dr John Parslow
CSIRO Marine Laboratories, Hobart |
Publication Details
Published by:
Land and Water Resources Research and Development Corporation
GPO Box 2182 Canberra ACT 2601
Telephone: (02) 6257 3379
Facsimile: (02) 6257 3420
Email: public@Iwwrrdc.gov.au
WebSite: www.lwrrdc.gov.au
© LWRRDC
Published Electronically on au.riversinfo.org by the Environmental
Information Association (Incorporated) with the permission of LWRRDC and
Environment Australia. Environment Australia assisted by providing copies
of the manuscript for electronic publication. The Natural Heritage Trust
provided project funds which were used to assist in publishing this material. In the case of variation between this document and the hard copy original the original takes precedence. (Bryan Hall).
Disclaimer:
The information contained in this publication has been published by
LWRRDC to assist public knowledge and discussion and to help improve the
sustainable management of land, water and vegetation. Where technical information
has been prepared by or contributed by authors external to the Corporation,
readers should contact the author(s), and conduct their own enquiries,
before making use of that information.
Publication data:
Estuarine Health Assessment Using Benthic Macrofauna. John Moverley & Alastair Hirst, Museum Victoria. LWRRDC Occasional Paper 18/99 (Urban Subprogram, Report #11) 1999.
Report No 11, LWRRDC Occasional Paper 18/99.
ISSN 1320-0992
ISBN 0 642 26768 5
Managing Agencies
-
The Urban Sub Program was managed by Water Services Association of Australia
under a contract with Land and Water Resources Research and Development
Corporation.
-
Environment Australia funded the research projects of the Urban Sub Program
of the National River Health Program.
-
Hard copy designed by: Nuttshell Graphics, North Melbourne
-
Hard copy printed by: Aristoc Offset, Mt Waverley
- Electronically Published by: Environmental Information Association (Incorporated)
Summary
- Objectives
- Methods
- Findings
- South-eastern Australian Estuarine Communities
- RIVPACS Model
- K-dominance Curves
- Recommendations
Objectives
The major objective of this project was to evaluate whether a RIVPACS-type approach could be used to assess estuarine environmental health in Australia. This required two questions to be answered, can RIVPACS-type models be constructed for Australian estuarine habitats and, if they can, are these of value in assessing environmental health.
Providing the data were available, a secondary objective was to evaluate other approaches to estuarine health assessment.
Methods
This study investigated macrobenthic communities of coastal plain estuaries, whether the mouths were open or closed. Included were a number of brackish coastal lagoons that were intermittently open to the ocean and had small streams flowing into them.
First a temporal study was conducted of 11 sites in four estuaries. Samples were collected by grab at approximately three monthly intervals. The first set of samples in this study was used as a pilot survey for the main study.
Second in the main survey, grab and dredge samples were collected from 89 sites in 29 mainland south-eastern Australian estuaries. South-eastern Australian estuaries were observed to have open or closed mouths, a halocline or to be well mixed vertically and to have a horizontal gradient from fresh water at the head of the estuary to salt water at the mouth or not to have a horizontal gradient. These factors were considered to have major impacts on the physicochemical environment of estuarine macrobenthos so were used in an a priori classification of estuaries. Five classes were recognised.
- Classical - unrestricted sea-water input and continuous inflow of a large volume of fresh water.
- Marine - unrestricted sea-water input but little freshwater input. Marine conditions exist throughout such estuaries.
- Homogeneous - little sea-water and freshwater input with the estuary having a shape that allows the water to be mixed by the wind. The salinity is the same throughout the estuary, and may be marine, but is most frequently diluted.
- Layered - little sea-water and freshwater input, and the water is poorly mixed by wind. A layer of low salinity water floats over a layer of high salinity water. The upper layer extends to the mouth, and the deep layer extends to the head of the estuary. The salinity of the deep layer is similar throughout the estuary, and may be sea water, but is most frequently diluted.
- Riverine - little sea-water input and relatively high freshwater input. Fresh water extends right to the beach where it flows over a berm and enters the ocean. Normally sea water input is only from waves breaking over the berm during rough seas. Although the water upstream of the berm is fresh, the presences of estuarine fauna suggest that biologically these are estuaries.
Findings
South-eastern Australian Estuarine Communities
Species – abundance profiles for grab and dredge samples were similar. Numbers of individuals in the samples were spread over four orders of magnitude. Numbers of taxa in the samples were low, 50% of samples had fewer than 10 taxa. For each method, approximately 40% of the total numbers of taxa collected were only from one site and 75% occurred in fewer than five samples. Two-way Table analysis revealed many species assemblages were represented in less than three samples, and several by just one sample. These observations are partly due to the low numbers of individuals in many samples, the low numbers of taxa that naturally occur at estuarine sites and the diversity of habitats present in the upper and lower reaches of certain estuarine types.
Differences in taxa present, community structure, numbers of individuals and numbers of taxa were observed between the macrobenthic communities of the different estuarine classes.
The macrobenthic communities of many estuarine sites were indicative of stressed environments, although there was no obvious anthropogenic impact at the sites. Presumably this is because of stress caused by natural environmental perturbations such as flooding and deoxygenation. Spatial and temporal fluctuations of environmental quality are probably natural characteristic of Australian estuaries because of their erratic freshwater input. The results suggest that natural variability of estuarine communities is large. It will therefore only be possible to identify large anthropogenic changes.
The temporal study revealed an order of magnitude change in density and large differences in species accumulation curves for the same sites. This means the probability for collecting different taxa varies with time. Any acceptable method for assessing estuarine health needs to be able to incorporate such variability in its interpretation of a site's health.
RIVPACS Model
RIVPACS (River Invertebrate Prediction and Classification System) models were developed in the early 1980s to assess river health in the United Kingdom (Moss et al. 1987). As well as the United Kingdom, RIVPACS-type models (AusRivAS) are now being used throughout Australia for river health assessment.
RIVPACS-type models produce a list of taxa and the probability they will be collected from a site. The number of species expected to be in a sample can be calculated from the probabilities. The ratio of the observed taxa to expected taxa (O/E) is then used as an index to environmental health. A value of one indicates the expected community is present. A value that deviates from one, either greater or less, indicates some disturbance has changed the community.
We demonstrated that a RIVPACS-type model could be developed for south-eastern Australian estuaries. However, its usefulness is questionable. The sensitivity of a RIVPACS-type model depends on the numbers of taxa predicted to occur at test sites, which have a probability of being collected greater than 0.5. Our model predicted between 4 and 13 taxa for test sites. This is at the lower limits for the model to be acceptable for monitoring environmental health. Another difficulty revealed by the study is that temporal changes in estuarine macrobenthic community is that the probability of collecting taxa may change with time.
K-dominance Curves
For the reference samples, multivariate analysis was applied to community variables, mostly derived from the sample's k-dominance curves. The ordination plot was divided into areas where the samples indicated a healthy environment, an unhealthy environment and questionable environmental health. Test sites can then be assessed by including their data in an analysis and seeing how they compare to the reference sites. The method is independent of species composition and sampling methods so can be used for a number of surveys and outside of the area where the reference samples were collected.
The method was shown to be useful for detecting changes to environmental health of the upper Derwent Estuary in Tasmania after improvements to waste water being discharged from a pulp and paper mill. It was also shown to be useful in monitoring changes to the macrobenthic community of the Calliope Estuary in Central Queensland after severe flooding.
Recommendations
There is no accepted definition of the term estuary in Australia. People from different backgrounds place different interpretations upon what is an estuary. Before large-scale monitoring of estuarine health can be undertaken there is a need to establish exactly what environments are to be monitored.
The different classes of estuaries we used were shown to have different macrobenthic community characteristics. Rather than trying to assess estuarine health for all classes combined it would be more appropriate to assess health based on different estuarine classes. It is recommended an estuarine classification system based on physicochemical factors related to the environment of the benthos be established.
Some estuarine sites have communities indicative of highly stressed sites. If the natural factors responsible for highly stressed estuarine sites can be identified they could be incorporated into a RIVPACS-type model. However, at the present stage, any biomonitoring of Australian estuaries will have to accept a relatively high proportion of "false" reports of poor environmental health. The k-dominance method can allow for this if proportions of the samples indicative of different levels of environmental health are examined. Such an approach would be more accurate if reference samples were derived from similar estuaries in the region where tests were being conducted. It is recommended that a national database of k-dominance curve information be established for Australian estuaries.
For this study samples were taken from fixed areas of sediment and the entire fauna sorted and enumerated. Ideally samples need to be large enough to describe the community, but not so large that an inordinate amount of time is being spent sorting. Because of the wide range in estuarine densities finding an optimal sample size is difficult. To collect enough individuals from the sites with low densities, numbers exceeding 10,000 would be collected from the sites with high densities. Such samples would take a considerable proportion of the sorting time. It is recommended that future samples use a fixed count approach. In this method excessively large samples are collected. These are split into subsamples, which are sorted until a predetermined number of individuals have been identified. This reduces the time spent sorting samples with large number of individuals and increases the effort being spent on sorting samples with low densities.
There were very diverse macrobenthic assemblages at the mouths of open estuaries and the head of closed estuaries. Because these estuaries are relatively uncommon, and the estuaries studied were selected randomly, such habitats were poorly represented in our samples. It is recommended that in a large-scale monitoring program such habitats should be deliberately targeted.
We demonstrated that a RIVPACS-type model could be developed. However, its value in monitoring estuarine health is questionable because of the low numbers of taxa predicted to occur at test sites. It is recommended that refinements to the sampling methods be tested before developing a national sampling protocol.
How temporal changes of the estuarine macrobenthic community impact RIVPACS-type models and other methods of estuarine health assessment are not known. It is recommended that further studies be undertaken to document the relative significance of seasonal and year to year changes in Australian estuarine macrobenthos.
Because estuarine macrobenthic communities with low densities of individuals and few taxa occur naturally, it can not be assumed sites with communities indicative of a stressed environments have been subjected to an anthropogenic impacted. Multivariate analysis of k-dominance curves can be used to identify stressed sites. Detailed studies of these sites can then be undertaken to see if they are anthropogenically stressed. For regional and single estuaries k-dominance curves can be used to see if the proportions of sites with communities indicative of poor or questionable environmental health are changing. We therefore believe the multivariate analysis of k-dominance curves is an appropriate method for assessing the health of estuarine sites, and recommend a database of reference sites for comparison of different estuary classes in different geographic locations be established.
Acknowledgements
We thank:
Penny Berents, Alan Jones, Richard Marchant, Gary Poore and Robin Wilson. These were members of the Estuarine Health Project steering committee, who assisted with the input of ideas for designing and implementation of the study and analysis of the data. We would particularly like to acknowledge the assistance of Richard Marchant in developing the RIVPACS-type model. We would also like to thank Gary Poore for his assistance in the day to day running of the project.
Jill Bottomley, Alan Jones, Tim O'Hara, Gary Poore, Jo Taylor, Genefor Walker-Smith and Robin Wilson who read and commented upon the manuscript.
Karl Inne Ugland of University of Oslo, Biological Institute who made the program Cumulate for calculating species accumulation curves available.
J. Simpson and R. Norris of the Co-operative Research Centre for Freshwater Ecology for their advice in constructing the RIVPACS-type model and assistance in running our data.
Fletcher Challenge Paper for providing macrobenthic community data for the upper and mid-reaches of the Derwent Estuary.
Specialist identifications were made by Dr A. Pinder (oligochaetes), Dr R. Wilson (polychaetes), Dr E. Wallis (mysids and ostracods), Ms J. Taylor (carids and penaeids), Dr G. Poore (other crustaceans), Dr R. Marchant (insects), Dr W. Ponder (molluscs), Mr T. O'Hara (echinoderms) and Ms T. Bardsley (fish). We appreciate the assistance these people gave.
This project was funded from the National River Health Program, Urban Research and Development Sub-program Project entitled "Estuarine Health Assessment using Benthic Macrofauna".
Chapter 1: Introduction
- 1.1 Defining Estuarine Habitat
- 1.2 Estuarine Health Assessment
- 1.3 Sampling Methods
- 1.4 Estuarine Community Characteristics
Locally, regionally and nationally, estuaries play important roles in recreation and tourism (boating, fishing, swimming, bird watching and aesthetic appreciation), primary production (fisheries and aquaculture), commerce (transport, natural harbours) and industry (cooling water, waste disposal) (Bowden 1996; Lauff 1967). Because of this estuaries are focal points for urban, industrial and rural development.
Estuaries are also important for wildlife. Most estuaries are highly productive, supporting dense populations of invertebrates, fish and birds. Estuaries are important nursery areas for many commercial fish species. The dense populations of fish and invertebrates normally found in estuaries support high densities of waterbirds (ducks, swans, pelicans, cormorants etc) and the intertidal areas provide feeding grounds for migratory and resident waders (curlews, sandpipers, plovers, egrets, etc).
Freshwater run-off from river catchments enters the sea through the estuary. Within the estuary there is a mixing of fresh water and sea water. As the salinity increases, there are major changes in water chemistry. This leads to the precipitation of particulate matter and the concentration of chemicals carried down the river from the catchment area. Because of the concentration of nutrients, estuaries are usually highly productive. The same processes that concentrate nutrients in the estuary can also concentrate pollutants. Common sources of estuarine pollution are the disposal of industrial and urban waste, petrochemical spills from boating and acid run-off from wetlands that have been reclaimed for urban and industrial development. Habitat modifications through dredging, reclamation and changed freshwater inputs have also resulted in the degradation of estuarine environments. Catchment clearing and dam construction impairs the "self-cleaning" of estuaries by changing the flushing rates caused by flooding.
Because of their high level of human use and their susceptibility to catchment disturbances, estuaries are easily degraded. Australia is presently becoming aware of these problems and implementing local and catchment level programs to maintain and improve estuarine health. Management tools are needed to assess where such projects need be undertaken and to assess their success. This report evaluates methods for determining estuarine health using benthic macrofauna i.e., the communities of invertebrates living in the mud or sand of the estuary floor. Benthic invertebrates have frequently been used as indicators in studies of this sort because the animals involved are relatively immobile (unlike fish), can be reliably identified, can be repeatedly sampled, and are responsive to known pollutants. The health of benthic communities is believed to be indicative of the other assemblages such as fishes or birds, in which the public shows a greater interest.
1.1 Defining Estuarine Habitat
Estuaries are the boundary between marine, freshwater and terrestrial ecosystems. They are poorly defined with two definitions being widely used.
- An estuary is where the tidal mouth of a river meets the stream (Day 1981; Ketchum 1983) i.e. coastal plain estuaries. This definition excludes river mouths that have closed bars and are therefore not tidal. Also, because of the ephemeral nature of Australian rivers, at times some will not have a stream.
- Estuaries are coastal indentations with restricted connections to the ocean that are at least intermittently open (Cameron & Pritchard 1963; Day et al. 1989). Included in the second definition are coastal plain estuaries, fjords and, depending on their shape, some gulfs, sounds and inlets (Bowden 1967). This definition is more appropriate for the Australian environment where there are closed bars, coastal lagoons and rivers that only intermittently have freshwater discharges, producing systems where salinity is similar throughout and may be greater, the same or less than that of sea-water.
With regards to the first definition it has been argued that tides are essential for an estuary and that some other term needs to be developed for brackish water bodies that are cut off from the sea (D. McLusky, University of Sterling, pers. comm.).
Under the second definition, embayments like Port Phillip Bay, Jervis Bay, Botany Bay and Moreton Bay are estuaries. In such inlets, there is little influence of freshwater input, and the fauna is identical to that of coastal embayments without restricted connections (P.A. Hutchings, Australian Museum, pers. comm.). For sampling of fauna in these "estuaries", the most appropriate methods are the same as those used for coastal embayments i.e., large sampling gear that needs to be deployed from fishing boats or research vessels. In many south-eastern Australian coastal plain estuaries, choice of sampling gear is restricted because of the need to use boats that can navigate through water that is frequently less than 0.5 m deep and sometimes as shallow as 10 cm. From the perspective of national environmental health assessment, these large "estuaries" should be examined within a study of marine embayments rather than along with coastal plain estuaries. In the United Kingdom there are legal and financial implications on whether wastewater is being discharged into an estuary or coastal water. With regards to such bays, they have adapted the concept that such bays are only estuaries if their salinity is 95% less than sea water for at least 95% of the time (Elliott, 1997).
As well as the problems of identifying which bodies of water are estuaries there are two definitions for the upstream limit of an estuary. One definition is the highest point to which the tide reaches, the other is the highest point to which salt water is detectable. The first includes "tidal freshwater" but the second does not (McLusky 1993).
Australia has not yet legally defined an estuary, and until this is done it is not possible to limit the habitat an estuarine health study needs to cover. This project concentrates on coastal plain estuaries.
1.2 Estuarine Health Assessment
Methods for the assessment of estuarine health are necessary to facilitate monitoring of ecosystem management programs, compliance with water discharge permits and the detection of pollution. Thus they will assist with the management of estuaries and the maintenance of long-term estuarine health. Methods that allow regional comparisons are preferable because local changes can then be compared to regional patterns. It was with the objective of developing regionally comparable protocols for the monitoring of estuarine health that this work has been carried out.
RIVPACS (River Invertebrate Prediction and Classification System) models have been used to determine environmental health of rivers in the U. K. since the early 1980s (Moss et al. 1987) and more recently in Australia (Marchant et al. 1997; Parsons & Norris 1996). With the exception of some applications within lakes (Johnson & Wiederholm 1989; Reynoldson et al. 1995), few attempts have been made to apply these models to other ecosystems. Our primary effort has been to attempt to produce predictive models for estuarine habitats in south-eastern Australia.
We first sought existing data sets of subtidal macrobenthic communities from south-eastern Australian estuaries. In Victoria, data were available from unpublished studies of the Hopkins Estuary and Mallacoota Inlet. Papers had also been published on two Victorian estuaries, the Yarra and the Gippsland Lakes (Poore 1982; Poore & Kudenov 1978) but the species abundance data from these studies have been lost. Data were also available for Port Phillip Bay. For New South Wales, data were available for the Hawkesbury Estuary, Botany Bay and Pittwater. Port Phillip Bay, Botany Bay and Pittwater data were not considered as suitable because these are not coastal plain estuaries. A study of estuaries was conducted in the Eurobodalla Shire (Briggs 1980) but only intertidal macrofauna had been systematically sampled.
Different methods were used to collect the samples in the three studies of coastal plain estuaries for which data were available. This was a serious impediment to their use in evaluation of health assessment methods, particular for a RIVPACS-type model where standard sampling methods are required. For the appropriate analytical methods our project depended on collection of a new data set using standard sampling methods.
1.3 Sampling Methods
To determine which gear provides the most reliable samples for estimating estuarine health, samples were collected by grab and by dredge.
Grabs penetrate the sediment to collect infauna. They are quantitative in that a known surface area is sampled. However, because the type of sediment affects penetration depth, over a wide range of sediment types different volumes are sampled. Thus faunal densities collected from different sediment types are not strictly comparable.
Dredges penetrate a few millimetres, collecting from the sediment surface and just above. They are better for collecting epifauna than grabs. Because dredge samples come from a larger area, they amalgamate the patchiness evident in grab samples. Also, because of the larger area sampled, dredges would be expected to collect more rare species and thus could provide better presence/absence data than grabs. Theoretically, dredges can sample a fixed area if towed for a set distance. In practice, this was not possible because of the variation in boat speed due to tides, currents and wind. Even if the sled is towed for the same distance, dredge samples from different habitats may not be quantitative because the net becomes blocked with algae, seagrass and detritus. The quantities of these that exist in a location will affect the collecting efficiency of a dredge.
1.4 Estuarine Community Characteristics
The unpredictable abiotic changes of estuarine environments select for species that can live and reproduce over a wide range of environmental conditions i.e. generalist species. This leads to estuarine communities generally having lower species diversity than marine or freshwater environments. To adjust to environmental variability many estuarine animals have diverse feeding strategies. This decreases the probability of ecological process being lost from the community if species become locally extinct due to environmental fluctuations. Consequently, although taxonomic diversity of estuarine communities may be low, functional diversity is usually relatively high and two estuarine assemblages with different taxa may functionally be the same (Costanza et al. 1996).
The low species diversity, diverse feeding strategies of estuarine animals and high productivity of estuaries result in exceptionally high densities of individuals at some sites. The highest density recorded in this survey was the gastropod Ascorhis tasmanica, with a density of 11,052 individuals.m-2. Usually, extremely large numbers of a few species dominate estuarine assemblages. The properties of water and the physical processes occurring in the estuary mean estuarine animals are highly mobile. Because of the high mobility, animals that are breeding in exceptionally high densities at some sites, will have individuals widely dispersed throughout the entire estuary, including sites they are not ecologically adapted for. As an individual in a sample may only have been recently transported to the sampling location, use of presence/absence data for estuarine animals may be of limited value for indicating whether a species is successfully living at a site. This may need to be incorporated into models that are being used to assess estuarine health.
Chapter 2: Estuarine Environments
- 2.1 Estuary Mouth
- 2.2 Vertical Mixing
- 2.3 Horizontal Mixing
- 2.4 Estuary Models
Deeley and Paling (1999) define and discuss the concept of estuarine health. Estuarine health studies using macrobenthic communities require the detection of change when the community varies from that of a healthy site. To do this it is necessary to be able to predict or describe communities that occur at healthy sites. Recognition of different estuarine types could be important because communities in one estuarine type may be naturally different from those in other types of estuaries. Thus, separating different estuarine types may reduce variability in the data allowing more accurate predictions to be made. Consequently, an aspect of estuarine macrobenthic communities that required investigating was if south-eastern Australian estuaries could be divided on physicochemical properties into subgroups characterised by different macrobenthic communities.
Digby et al. (1998) developed a classification of Australian estuaries based on a suite of physical characteristics and showed these could be used to make predictions about the intertidal vegetation. They did not test their model on subtidal macrobenthic communities. Their classification divided Australian estuaries into 11 classes, with all south-eastern Australian estuaries falling into the class defined by a temperate climate and low tidal range.
An alternate morphological classification (Roy 1984) divides south-eastern Australian estuaries into drowned river valleys, barrier estuaries and saline coastal lakes. The major factor characterising these three types is the height and development of the sill/sand barrier at the mouth of the estuary (Fig. 2.1). Because the sill/sand barriers build up and break down, an individual estuary may fall into different classifications at different times.
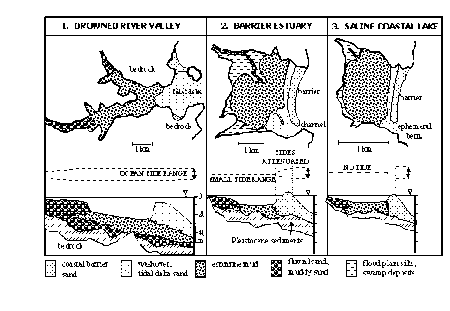
|
|
Figure 2.1 Three main south-eastern Australian estuarine morphological types showing idealised sediment distributions in plan and section with approximate dimensions. Tidal ranges in the estuaries are shown in relation to the open ocean. From Roy 1984.
|
|
(original size image)
|
Our field observations suggested estuaries in south-eastern Australia varied in ways not included in these descriptions. To explain environmental differences observed in the estuaries an a priori classification based on three factors was developed. 1) The mouth may be open or closed and this regulates tidal mixing and frequency of input of salt water. 2) presence of a halocline, which indicates poor vertical mixing of the water. 3) presence of a horizontal salinity gradient, which indicates mixing of fresh water and salt water along the estuarine gradient.
2.1 Estuary Mouth
Whether an estuary mouth is open or closed has a major influence on the physicochemical environment of the estuary. A number of factors interact to make the mouths of south-eastern Australian estuaries susceptible to closing.
- Most south-eastern Australian estuaries discharge into high-energy surf areas, where large volumes of sand are moved along the coast by offshore currents and along the beach by wind. Therefore, sand deposition in the estuary mouths may be high.
- The estuaries are shallow because of tectonic stability and low coastal relief (Eyre 1998). Many therefore require little sand to fill them in.
- Tidal range in southern New South Wales and Victoria is small with extreme ranges being between 1 and 2 m (Bucher & Saenger 1989). Consequently, tidal currents, particularly in the smaller estuaries, are weak. Thus tidal currents alone may not be sufficient to keep the river mouth open.
- The estuaries have highly variable and erratic discharges caused by the rainfall patterns (Roy 1984), short river lengths (Eyre 1998), and steep sided catchments (Briggs et al. 1980). The steep catchments and short river lengths means rainfall rapidly passes through the river system. The erratic discharge means there are irregular peaks and troughs in the river discharge rates. At times of low discharge, scouring out of the channel is reduced and sand may build up in the mouth. This sand, which can close the mouth, will only be cleared after a period of rainfall.
The impact of these factors varies with the river length, the tidal exchange volume and the morphology of the river mouth so that some rivers will be permanently open while others are normally closed.
During periods of low discharge, sand builds up and closes the mouth of an estuary. During periods of high discharge the barriers are breached. As a consequence of mouth closures, the salinity regime of many south-eastern Australian estuaries is driven not only by freshwater input but also by salt-water input (Roy 1984).
There is no clear demarcation between open and closed estuarine states. Physicochemical changes to the estuarine environment starts when the mouth is still open but the sill partially blocks the entrance channel so that tidal exchange is restricted. Many estuaries have sills at heights between high water neap and high-water spring, so that tidal flow only occurs during the higher tides. Even when the sill's height is greater than high-water spring, washover during periods of rough seas will put salt water into the estuary so that south-eastern Australian estuaries are rarely completely cut-off from the ocean.
Although most biological impacts of mouth closure are indirect through the physicochemical environment, there are some direct responses. For example, penaeid prawns have a marine planktonic life stage and can only settle in an estuary if it is open when they are metamorphosing. Thus two estuaries with identical physicochemical environments can have different communities due to differences that occurred several months prior to sampling. Such factors will influence estuarine health assessment if the methods are based on presence and absence of species.
2.2 Vertical Mixing
In a layered estuary, the benthic community may be limited by oxygen supply, which can only move from the surface layer to the deeper layer by diffusion. Toxic gases such as hydrogen sulphide and ammonia, released from the breakdown of organic material in the sediments, may also build up in the deeper waters. In non-stratified estuaries, turbulent mixing ensures that oxygen is carried to the benthos and toxic gases are carried to the surface where they can disperse into the atmosphere. The differences in the physicochemical environment for benthos in mixed and stratified estuaries should lead to significant differences between macrobenthic communities.
In closed estuaries, water movement and mixing can only be produced by wind and storm action (Morrisey 1994). The shape of the estuary greatly influences its potential for wind mixing. In our study, closed estuaries that were river shaped usually possessed a halocline, indicating they were poorly mixed. Closed estuaries incorporating a lagoon generally did not have a halocline, indicating such estuaries were well mixed. Presumably, the fetch of lagoons allowed significant mixing of water by wind action but in narrow estuaries this was insignificant.
In estuaries with a halocline, a layer of low salinity water usually extended to the mouth. An example of this was the Merriman Estuary where bottom salinity at a 0.2 m deep site on the bar was 8.30/00. A 2 m deep site, 50 m upstream from the bar, had a salinity of 15.80/00. Consequently, fauna living in shallow water near the mouth of estuaries with a halocline must be tolerant of low salinities. It is not known if there is a stable euryhaline community in this habitat or if there is a change in the community after the bar has closed and low salinity water penetrates into the lower estuary.
At deeper sites, a halocline has an indirect impact on the benthic community through oxygen stress (Eyre 1998). A number of sites sampled in layered estuaries were anoxic with only sparse fauna. However, this was not true of all sites with a halocline. It is possible that there is a time lag between formation of a halocline and the onset of anoxia, or that under some conditions there is still sufficient oxygen to support a diverse macrobenthic community.
Table 2.1: Depth and salinity data for the Aire Estuary, a closed estuary in central Victoria. |
Site | Distance From Mouth (m) | Salinity (ppt) | Depth (m) |
Bar | 0 | 5.4 | 0.2 |
Lower | 50 | 9.9 | 1.2 |
Mid-estuary | 1500 | 15.6 | 2.5 |
Upper estuary | 6300 | 17.8 | 4.0 |
2.3 Horizontal Mixing
In open estuaries, tidal movement and mixing of fresh and salt water is expected to produce a salinity gradient from marine water at the mouth to fresh water at the head. Most south-eastern Australian estuaries have closed mouths so they do not have a marked longitudinal salinity gradient (Fig. 2.2). Although rare, there were cases of bottom salinity increasing with distance from the mouth (e.g., the Aire Estuary, Table 2.1). In this case salinity appears to be related to depth instead of distance from the mouth. This however was atypical. Usually closed estuaries had a layer of fresh water over a pool of salt water, with the bottom salinity remaining similar near the mouth and as far upstream as we could get. Bottom salinities in the closed estuaries ranged from marine to diluted sea water. Several of the tidal estuaries we sampled also showed no salinity gradient being greater than 30 0/00 from the mouth to the head. Presumably this was due to there being little freshwater discharge at the time of sampling.
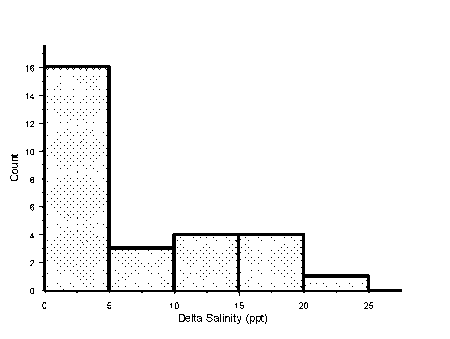
|
|
Figure 2.2 Frequency histogram of the difference between maximum and minimum salinities recorded from each estuary. This reveals that half of the estuaries in the study had a horizontal salinity gradient with less than 5 0/00 difference between the salinity at the mouth and head of the estuary.
|
|
(original size image)
|
2.4 Estuary Models
Because of low freshwater input and closed mouths, many south-eastern Australian estuaries do not fit the general model of estuaries. In this model fresh water flows into the head of the estuary, salt water is carried into the mouth of the estuary with the tide and there is a gradient of decreasing salinity with increasing distance from the sea. We believe five models apply to south-eastern Australian estuaries.
- Classical - unrestricted sea-water input and continuous inflow of a large volume of fresh water.
- Marine - unrestricted sea-water input but little freshwater input. Marine conditions exist throughout such estuaries.
- Homogeneous - little sea-water and freshwater input with the estuary having a shape that allows the water to be mixed by the wind. The salinity is the same throughout the estuary, and may be marine, but is most frequently diluted.
- Layered - little sea-water and freshwater input, and the water is poorly mixed by wind. A layer of low salinity water floats over a layer of high salinity water. The upper layer extends to the mouth, and the deep layer extends to the head of the estuary. The salinity of the deep layer is similar throughout the estuary, and may be marine, but is most frequently diluted.
- Riverine - little sea-water input and relatively high fresh water input. Fresh water extends right to the beach where it flows over a berm and enters the ocean. Normally sea water input is only from waves breaking over the berm during rough seas. Although the water upstream of the berm is fresh, the presence of estuarine polychaetes, amphipods and brachyurans suggest biologically such habitats should be considered an estuary. Only one of these "estuaries" was sampled.
Although river discharge into many south-eastern Australian estuaries is erratic, evaporation rates are never high enough for hypersaline estuaries to develop (Morrisey 1994).
Western Victoria has a Mediterranean climate and the estuaries open during the winter wet season and close during late spring or summer. Eastern Victoria and southern New South Wales have rainfall distributed evenly throughout the year. In this region, the estuaries are opened naturally by recurrent flooding throughout the year (Bird 1967). Although most of the estuaries open and close within a year or less, there are examples of longer periods. The freshwater estuary, the Darby, had only been opened once in the past 10 years, and Wingan Inlet, a marine estuary, has only been closed once in the past 30 years.
Superimposed on the natural opening and closing of the estuaries are the artificial openings. In the 1970s, professional fisherman would open many of the southern New South Wales estuaries several times a year (Briggs et al. 1980). Many of these estuaries are in National Parks and management has reduced the number of times they are artificially opened. Regular artificial opening appears to have a big impact on the fauna. The Merriman Estuary had the greatest species richness for a layered estuary. This estuary is regularly opened to prevent salt water contaminating the river from which the town of Seaspray pumps its water. Presumably the regular opening of the estuary prevents conditions in the lower layer deteriorating to the point where they limit community development. This would indicate a time lag between closure of the river mouth and impact on the benthic community.
The different estuarine classes are subdivisions of a gradient dependent on freshwater and salt-water input. Freshwater input depends on past rainfall. Whether the mouth is open depends on past rainfall, sea conditions and tidal cycles. Consequently, the classifications we have presented are not static and at different times, a particular estuary may fall into different categories. The physicochemical environments observed for different estuaries may represent stages between the conditions associated with two estuarine classes. In response to the changing physicochemical environment, the macrobenthic communities will also be in a state of flux. Community changes will be gradual and probably occur some time after the mouth closes. Allowance for these changes need to be incorporated into the methods of assessing the health of an estuary.
The opening and closing of the mouth provides an opportunity for the fortuitous entrapment of species. An example of this was observed in the Coila Estuary. This was a closed estuary where tanaids formed a large proportion of individuals at all three sites. Tanaids are marine animals and generally do not occur in estuaries (Edgar et al. 1998). It is also probable that in some estuaries entrapment of large numbers of fish, which prey on macrobenthos, will occur. Such an event would presumably result in a macrobenthic community different from an estuary without such predators. To understand the impact of such events and successfully incorporate them into an estuarine health monitoring program requires temporal studies from a number of estuaries.
Chapter 3: General Material And Methods
- 3.1 Estuary Selection
- 3.2 Site Selection
- 3.3 Grab samples
- 3.4 Dredge Samples
- 3.5 Sample Processing
- 3.6 Abiotic Variables
- 3.7 Data Manipulation
- 3.8 Temporal Study
In the project, two studies of south-eastern Australian estuarine macrobenthos were carried out. These were an intensive study of 29 estuaries throughout south-eastern Australia and a temporal study of four estuaries widely separate in the region. The same methods were used in both studies.
3.1 Estuary Selection
The study was limited to south-eastern mainland Australia between the Victorian – South Australian border and Batemans Bay, New South Wales. Due to the high probability of anthropogenic impacts on the macrobenthos, estuaries with catchments in Melbourne and Geelong i.e. between Western Port and Barwon Heads were omitted. We recorded 96 estuaries in the study area. Only 49 of these estuaries were considered for this study because physical data suitable for building a RIVPACS-type model was not available for the other 47.
Bucher and Saenger's (1989) catalogue of Australian estuaries listed 49 estuaries in the study region compared to 96 recorded by us. This is partly because we recorded a number of small estuaries not included by Bucher and Saenger but the disparity was mostly due to differences in how we defined an estuary.
Bucher and Saenger defined an estuary as starting where the distance between opposite banks (including any intertidal land) first narrows to 2 km when approached from seaward. With their definition multiple discharge estuaries only occur where no distance between banks downstream from the river's joining is less than 2 km. Usually rivers in compound estuaries (i.e. where more than one river discharges through an estuarine mouth) come together where the banks are less 2 km apart. Bucher and Saenger consider these compound estuaries as a single entity.
Because run-off from the different catchments may have different impacts on the macrobenthos in the upper and mid-reaches of the different streams within a compound estuary, separation of compound estuaries was seen as being important for estuarine health studies. Also, separating compound estuaries into separate entities allows estuarine management to become part of an overall catchment management plan. Therefore, we have adopted a definition of an estuary, as being those sections of streams that flowed through the terrestrial coastal zone i.e. a coastal plain estuary. Using this definition, compound estuaries were treated as multiple estuaries. Marine embayments such as Port Phillip Bay are not included in this study because they are not coastal plain estuaries.
A pilot study was conducted in four estuaries, which were selected on geographic distribution, one from each end of the study region and the other two approximately one third from either end. The pilot study provided the first set of samples in the temporal study. These four estuaries were sampled again as part of the overall study. Based on stratification by catchment area, 26 estuaries were selected for sampling from the 45 estuaries suitable for the study. These are the 49 estuaries in the study area with data suitable for use in developing a RIVPACS-type model minus the four estuaries sampled in the pilot survey.
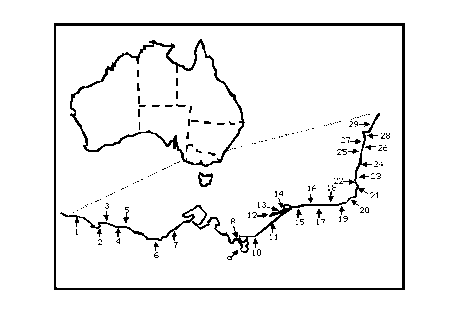
|
|
Figure 3.1: Location of estuaries sampled in south-eastern Australia. Numbers are the estuary numbers (EN) in Table 3.2.
|
|
(original size image)
|
Catchment area, which was found not to show any trends with geographic location, was used as a means of randomising the estuaries. Estuaries were ranked by decreasing catchment area and within each successive ranked group of five, the first, second and fourth were selected for sampling. One of the selected estuaries was not sampled because it was inaccessible. Between 11 March and 16 April 1997, grab and dredge samples of macrobenthos were collected from 90 sites in 29 Victorian and southern New South Wales estuaries (Fig.3.1).
3.2 Site Selection
Usually samples were collected from three locations in each estuary. The objective was to sample an upper, mid- and lower estuarine site. Sites were selected after a visual inspection of the estuary. The lower estuarine sample was normally taken behind the sill at the river bar. The distance from the bar varied from several metres to a few hundred metres depending on the size of the estuary.
If given a choice, the upper estuary site was located where grasses and trees were growing in the water. In many estuaries, we could not reach this point because we could not get that far up the estuary or there was no soft sediment for sampling because the upper estuary bottom was rock, cobble, hard clay, etc. In such cases, a decision based on location, surrounding vegetation, salinity, etc was made as to whether the site was in the mid- or upper reaches. In these cases, where vegetation suggested the site was still mid-estuarine, two mid-estuarine samples were collected.
The third site was usually located approximately half way between the lower and upper estuarine sites. However, if there was some obvious habitat change in the estuary above the lower reaches, the mid-estuary samples were taken there. In estuaries where there were less than three obviously different habitats along the estuarine length, a sample was taken at the bar, another from relatively close to the mouth, and the third from the furthest upstream, accessible site. In coastal lagoons, where the only habitats were the bar and lagoon body, only two samples were collected.
3.3 Grab samples
Three methods were used to collect samples in the grab set. Most of the samples were collected with a 15 x 15 x 15 cm Ekman grab, which was lowered to the bottom by a cable. For sites between 0.5 and 1.25 m deep, where there was dense seagrass, samples were collected with the same Ekman grab attached to a handle. At sites less than 0.5 m deep, the substrate was usually too compact for an Ekman grab and a corer of similar surface area (15.5 cm diameter) was used. The corer was pushed into the substrate to a depth of 15 cm. At each site, five grabs were collected and combined to give a single site sample representing 0.1125 m2. Samples collected in the corer were standardised to represent a 0.1125 m2 area.
3.4 Dredge Samples
Dredge samples were collected with a scaled down Woods Hole macrobenthos sled with a 30 cm wide mouth. Dredge samples were collected by towing the sled approximately 300 metres. At sites where the water depth was less than 30 cm the dredge was towed by wading through the water. At deeper sites it was towed behind the boat while rowing or drifting. Depending on conditions, the sample was usually made up of three tows of approximately 100 m each, which went back and forth over the sample site, or in a triangle around the site. Where there was a large open expanse and the sediment was such that there was little chance of losing the sample, a single tow was made. Where weed rapidly filled the net, or the sediment was so soft that the dredge readily became bogged, a greater number of smaller samples were taken.
3.5 Sample Processing
Directly after collecting, grab and dredge samples were washed through a 1 mm mesh sieve and the retained fraction stored in a polyethylene bag. After all the sites within an estuary had been sampled and we had returned to shore, the samples were fixed in borax buffered sea-water formalin. After being fixed in formalin for at least 24 hrs, the samples were again washed through a 1 mm mesh sieve and then stored in 70% ethanol.
Macrobenthos was extracted by washing the fixed samples through a 1 mm mesh sieve until all fine sediment was washed away. Then the remaining material was sorted under a dissecting microscope. Fauna was sorted to major taxon and then specimens identified to lowest possible taxon and enumerated for each sample. Specialist identifications were made by Dr A. Pinder (oligochaetes), Dr R. Wilson (polychaetes), Dr E. Wallis (mysids and ostracods), Ms J. Taylor (carids and penaeids), Dr G. Poore (other crustaceans), Dr R. Marchant (insects), Dr W. Ponder (molluscs), Mr T. O'Hara (echinoderms) and Ms T. Bardsley (fish).
Table 3.1 Environmental variables and units of measurement. Superscripts refer to the method of data acquisition and are listed below the table. |
Site Variables |
Physicochemical1 |
Sediment2 |
Sediment Redox (mV) |
Mean particle size (phi) |
Water temperature (°
C) |
Sorting coefficient (phi) |
Surface salinity (o/oo) |
Interstitial salinity (o/oo) |
Bottom salinity (o/oo) |
Sediment organic carbon (%) |
Salinity differential (bottom – surface) (o/oo) |
Clay (%) |
Maximum Depth (m) |
Silt (%) |
| |
Gravel (%) |
| |
Sand (%) |
Sample Variables3 |
Seagrass dry weight (gm) |
Algal dry weight (gm) |
Estuarine Variables |
Latitude (decimal degrees)4 |
Longitude (decimal degrees)4 |
Ranked position along the coastline from the Glenelg R. to Batemans Bay4 |
Catchment Land Use (4 categories)5 |
Catchment area (km2)6 |
Estuary mouth (open or closed)7 |
Estuary shape (maximum estuary width (m)/ mean estuary site depth (m))8 |
1. Recorded at time of sampling.
2. Sediment sample taken at each site and analysed by MAFRI Queenscliff, Vic.
3. Seagrass and algae from grab sample dried at 60 °
C for approx. 24 hours.
4. Information derived from 1:100,000 maps.
5. Catchment land use categories derived from information within Saenger and Bucher (1989) and 1:100,000 maps.
6. Obtained from Victorian discharge records, Bucher and Saenger (1989) and 1:100,000 maps.
7. Direct observation.
8. Calculated by dividing the maximum estuary width (taken from 1:100,000 map) by the mean maximum depth of the sites sampled in the estuary.
3.6 Abiotic Variables
Abiotic variables are listed in Table 3.1. Sediment samples were collected using the same method as the fauna samples and approximately 250 ml of the sediment retained for later analysis. Sediment samples were kept frozen until they could be analysed by the Marine and Freshwater Resources Institute at Queenscliff. Particle size was analysed by an automated settling tube system and carbon content was determined by digestion of the organic material in the samples and measuring the amount of carbon dioxide produced. Salinity of the interstitial water in the sediment sediments was also measured.
Table 3.2 Sampling Sites, their Ranked Position (RP), Site Number (SN) used throughout the report and the estuary number (EN) for the locations given in Figure 3.1.
|
RP | SN | EN | Location | RP | SN | EN | Location | RP | SN | EN | Location |
1 | 49 | 1 | Glenelg, Lower | 31 | 17 | 11 | Tarra, Lower | 61 | 68 | 20 | Wallagaraugh, Mid 1 |
2 | 50 | 1 | Glenelg, Mid | 32 | 16 | 11 | Tarra, Mid | 62 | 69 | 20 | Wallagaraugh, Mid 2 |
3 | 51 | 1 | Glenelg, Upper | 33 | 15 | 11 | Tarra, Upper | 63 | 67 | 20 | Wallagaraugh, upper |
4 | 59 | 2 | Surrey, Bar | 34 | 20 | 12 | Merriman Ck, Bar | 64 | 64 | 21 | Towamba, Lower |
5 | 58 | 2 | Surrey, Lower | 35 | 19 | 12 | Merriman Ck, Lower | 65 | 65 | 21 | Towamba, Mid 1 |
6 | 60 | 2 | Surrey, Mid | 36 | 18 | 12 | Merriman Ck, upper | 66 | 66 | 21 | Towamba, Mid 2 |
7 | 56 | 3 | Fitzroy, Lower | 37 | 26 | 13 | Gippsland Lake, Lower 1 | 67 | 61 | 22 | Nullica, Lower |
8 | 57 | 3 | Fitzroy, mid | 38 | 23 | 13 | Gippsland Lake, Mid 1 | 68 | 63 | 22 | Nullica, mid |
9 | 55 | 3 | Fitzroy, upper | 39 | 24 | 13 | Gippsland Lake, Mid 2 | 69 | 62 | 22 | Nullica, upper |
10 | 52 | 4 | Lake Yambuk, Lower | 40 | 25 | 13 | Gippsland Lake, Mid 3 | 70 | 71 | 23 | Curalo Lagoon, outlet |
11 | 54 | 4 | Lake Yambuk, Mid | 41 | 21 | 13 | Gippsland Lake, Upper 1 | 71 | 72 | 23 | Curalo Lagoon, body |
12 | 53 | 4 | Lake Yambuk, upper | 42 | 22 | 13 | Gippsland Lake, Upper 2 | 72 | 73 | 24 | Middle Lagoon, mouth |
13 | 48 | 5 | Moyne, Mouth | 43 | 28 | 14 | Tambo, Mid | 73 | 74 | 24 | Middle Lagoon, body |
14 | 46 | 5 | Moyne, Mid 1 | 44 | 27 | 14 | Tambo, Upper | 74 | 78 | 25 | Bilba Bilba Ck, Lower |
15 | 47 | 5 | Moyne, Mid 2 | 45 | 30 | 15 | Lake Tyers 2 | 75 | 77 | 25 | Bilba Bilba Ck, Mid? |
16 | 1 | 6 | Hopkins, Lower | 46 | 31 | 15 | Lake Tyers 3 | 76 | 76 | 25 | Bilba Bilba Ck, upper |
17 | 2 | 6 | Hopkins, Mid | 47 | 29 | 15 | Lake Tyers 1 | 77 | 88 | 26 | Lake Brou, Mid 1 |
18 | 3 | 6 | Hopkins, Upper | 48 | 38 | 15 | Lake Tyers 4 | 78 | 89 | 26 | Lake Brou, Mid 2 |
19 | 42 | 7 | Aire River, bar | 49 | 34 | 16 | Snowy River, Lower | 79 | 87 | 26 | Lake Brou, upper |
20 | 43 | 7 | Aire River, mouth | 50 | 32 | 16 | Snowy River, Mid | 80 | 86 | 27 | Coila River, lower |
21 | 44 | 7 | Aire River, mid 1 | 51 | 33 | 16 | Snowy River, Upper | 81 | 85 | 27 | Coila River, mid |
22 | 45 | 7 | Aire River, mid 2 | 52 | 36 | 17 | Yeerung, Sand Bar | 82 | 84 | 27 | Coila River, upper |
23 | 39 | 8 | Aireys Inlet, mouth | 53 | 37 | 17 | Yeerung, Lower | 83 | 80 | 28 | Tamago Lower,1 |
24 | 40 | 8 | Aireys Inlet, Mid, 1 | 54 | 35 | 17 | Yeerung, Mid | 84 | 81 | 28 | Tamago Lower,2 |
25 | 41 | 8 | Aireys Inlet, Mid 2 | 55 | 90 | 18 | Bemm River, Mouth | 85 | 82 | 28 | Tamago Mid |
26 | 14 | 9 | Shallow Inlet, Mid | 56 | 91 | 18 | Bemm River, Lower 1 | 86 | 83 | 28 | Tamago upper |
27 | 13 | 9 | Shallow Inlet, Upper | 57 | 92 | 18 | Bemm River, Lower 2 | 87 | 5 | 29 | Clyde, Lower |
28 | 10 | 10 | Darby, Lower | 58 | 70 | 19 | Wingan Inlet, mouth | 88 | 4 | 29 | Clyde, Mid |
29 | 12 | 10 | Darby, Mid | 59 | 7 | 19 | Wingan Inlet, Lower | 89 | 79 | 29 | Clyde, Mid 2 |
30 | 11 | 10 | Darby, Upper | 60 | 8 | 19 | Wingan Inlet, Mid | | | | |
For RIVPACS-type models of rivers geographic position has been found to be an important factor in determining species composition of a site. The shape of the south-eastern Australian coastline results in poor geographic separation of sites by latitude or longitude. Latitude places east – west separated Victorian sites together. Longitude places north – south separated eastern Victorian and New South Wales sites together. Rank Position along the coast from the Victorian/South Australian border to Batemans Bay (Table 3.2) was seen as the best variable to express geographic closeness of sites and has therefore been used as an abiotic variable in the RIVPACS-type modelling.
3.7 Data Manipulation
The following taxa were excluded from the analysis: juveniles of Amphioplus, Corophium, Fish, Gobiidae, mysids, Penaeidae, and Serpulidae because the individuals were too small for species level identification. Also discarded were the harpacticoids and nematodes. These were considered to be meiobenthic taxa, the majority of which would have been lost through the 1 mm mesh sieves used in this survey. Ascidians and cnidarians were also not used in the analysis, as taxonomic expertise for species level identifications was not readily available and many of these individuals were associated with secondary structures in the samples e.g. tree branches and seagrasses.
3.8 Temporal Study
Temporal samples were collected from four estuaries selected on geographic distribution, one near each end of the study region and the other two approximately one third from either end. The estuaries were the Hopkins (estuary number 6) at Warrnambool, the Darby (estuary number 10) on Wilsons Promontory, Wingan Inlet(estuary number 19) in East Gippsland, and the Clyde (estuary number 29) at Batemans Bay. The Hopkins Estuary was selected rather than the Glenelg, which was the most western estuary, because of the opportunity to collaborate with work being conducted at the University of Warrnambool. Four sets of samples were collected (Table 3.3). The second set of samples took longer to collect because they were collected as part of the intensive study with the other 25 estuaries.
The first set of samples was used as a pilot study to assess sample methods and sample size requirements. In the first set of samples, the dredge did not work; so only grab samples were collected for the temporal study. From each site ten replicate Ekman grab (15 x 15 x 15 cm) samples were collected. Unlike the intensive study, where multiple grabs were combined to give a single sample, these replicates were kept separate. These replicates were processed using the same methods given above.
Table 3.3 Sampling Dates for the four sets of temporal study samples |
Survey |
Sample dates |
Season |
1 |
12 – 20 Dec 1996 |
Summer |
2 |
10 Mar – 10 Apr 1997 |
Autumn |
3 |
7 – 17 Jul 1997 |
Winter |
4 |
28 Oct – 11 Nov 1997 |
Spring |
Chapter 4: Sample Profile
- 4.1 Sample Characteristics
- 4.1.1 Results
- 4.1.2 Discussion
- 4.2 Abiotic Effects on Abundance and Diversity
- 4.2.1 Salinity
- 4.2.1.1 Results
- 4.2.1.2 Discussion
- 4.2.2 Vertical Stratification
- 4.2.2.1 Results
- 4.2.2.2 Discussion
- 4.2.3 Seagrasses
- 4.2.3.1 Results
- 4.2.3.2 Discussion
- 4.2.4 Algae
- 4.2.4.1 Results
- 4.2.4.2 Discussion
- 4.3 Estuarine Categories
- 4.3.1 Results
- 4.3.2 Discussion
- 4.4 Classification of sample sites
- 4.5 Species Accumulation Curves
- 4.5.1 Results
- 4.5.2 Discussion
- 4.6 Conclusions
4.1 Sample Characteristics
Ideally, samples should be unbiased and quantitative so that they accurately reflect the communities from which they were collected. Such samples would readily allow the comparison of abundances for macrofauna and community structure over a range of habitats. However, all sampling methods possess some inherent biases, which vary with habitat and sediment type.
Grab and dredge samples have been collected for this project. The grab is biased towards slow moving epibenthic animals and other animals buried in the upper layers of the sediment. Motile epifauna will be poorly sampled because they can avoid a grab. The depth to which the grab penetrates depends on sediment type. Hence, for different sediments different volumes have been collected.
Dredges move across the bottom scraping a surface layer off the sediment. They collect fauna on or just under the surface, swimming just above the sediment and sheltering in weeds. They have a bias for collecting epifauna and should be more successful at collecting motile animals than a grab. However, highly mobile animals are able to avoid a dredge. Because the dredge only scrapes the surface, infauna are poorly sampled. The depth that the dredge penetrates depends on the sediment. For efficient use, dredges require that the water passes unhindered through the mesh of the collecting bag. When a large amount of weed or detritus blocks the net, samples are not collected with the same efficiency as from detritus free sediments. Thus efficiency of sampling infauna and epifauna varies with habitat.
To minimise sample variability due to between-site differences in substrate, the original proposal was to sample soft muds within the estuary channel at lower, mid- and upper estuarine sites. This was not practicable, because frequently such habitats were not available. Channels had substrates of gravel, cobble, rock, etc so samples could only be collected from the edge. The only substrate available in some estuaries was sand. In other locations the substrates were covered in algae or seagrass so that bare mud could not be sampled. Thus samples were collected from a wide range of substrates and depending on the ability of the grab to penetrate the various sediments different volumes would have been collected. Considering this, we believe the use of the corer for sampling the compacted sands was justified.
Profiles for the dredge and grab samples are compared to establish which is the most appropriate method to collect samples for assessing estuarine health. Important factors are the numbers of individuals in samples, species richness (i.e. the number of taxa), and the time required for extracting and enumerating the fauna.
4.1.1 Results
Because of the different sample biases of the grab and dredge, there is very little relationship between the numbers of individuals and species richness for samples collected from the same sites by the two methods (Fig. 4.1).
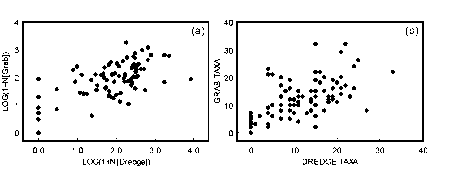
|
|
Figure 4.1 Scatterplots of the relationships between a) Log(1 + N) transformed numbers of individuals in the dredge samples and Log(1 + N) transformed numbers of individuals in the grab samples collected from the same sites. b) Numbers of taxa in the dredge samples and numbers of taxa in the grab samples collected from the same sites.
|
|
(original size image)
|
Numbers of individuals in both grab and dredge samples covered four orders of magnitude (0 to 1,784 for the grab and 0 to 8,581 for the dredge). Numbers of individuals in dredge samples (median 118) were greater than for the grab samples (median 89). However there were similar proportions of grab and dredge samples with low numbers of individuals per sample (less than 75) and high numbers of individuals per sample (more than 350) (Fig. 4.2a). Twenty percent of the samples collected by both methods had fewer than 30 individuals and 10% of grab samples and 12% of dredge samples had more than 500 individuals.
A total of 267 taxa was collected during the survey, 197 in the grab samples and 188 in the dredge samples. The maximum number of taxa in a grab sample was 32 and 33 in a dredge sample. Plots for the cumulative percentage of samples versus number of taxa in the samples are near identical for the two methods except for a greater proportion of dredge samples with no fauna (Fig. 4.2b). Samples collected by both methods were characterised by a large proportion of the samples having low numbers of taxa. Twenty-five percent of the samples had fewer than 10 taxa.
Plots for cumulative percentage of taxa versus number of samples are identical for the dredge and grab samples (Fig. 4.2c). For each method, approximately 40% of the taxa were only collected in one sample and 75% of the taxa occurred in five or fewer samples.
Plots for the number of taxa versus number of individuals are almost identical for the dredge and grab samples (Fig. 4.2d). For samples with fewer than 20 individuals, there was a strong correlation between numbers of individuals and numbers of taxa (Fig. 4.2d). For samples with more than 20 individuals, there is no correlation with most samples having between 5 and 20 taxa per sample.
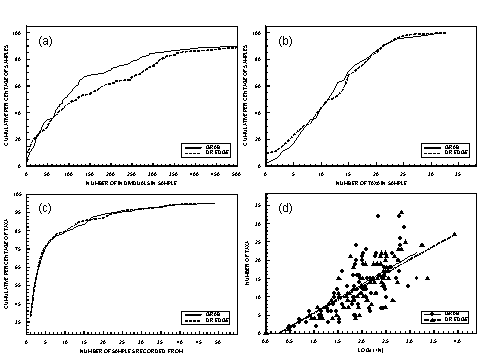
|
|
Figure 4.2 Profile for grab and dredge samples for all estuarine types combined. (a) Cumulative percentage of samples versus the number of individuals in the samples for samples with less than 500 individuals. (b) Cumulative percentage of samples against the number of taxa recorded in the samples. (c) Cumulative percentage of total taxa recorded versus the number of samples the taxa were recorded in. (d) Number of taxa against the number of individuals in the dredge and grab samples.
|
4.1.2 Discussion
Samples collected by both methods possessed near identical profiles. Numbers of individuals were spread over four orders of magnitude. Many of the samples had low numbers of individuals and many of the taxa were limited to only one sample and only a quarter of the taxa were found in more than five samples. These results reveal that neither method is inherently better for collecting samples for estuarine health assessment than the other. Sorting times for the two sets of data were similar so this study provides no justification for selecting one method rather than the other.
These results highlight several difficulties in sampling estuarine macrobenthos for assessing environmental health. A major difficulty is the large variation in the numbers of individuals and taxa in the samples. This makes it very hard to find an optimal sample size.
Samples with low numbers of individuals are highly susceptible to random variability. Community patterns for a site tend not to stabilise until at least 75 individuals in a sample have been sorted (J.H. Moverley, unpublished data). Thus, many of the samples collected for this project had insufficient numbers of individuals to effectively represent the community at the collection site. This could be overcome by increasing the sample size. Collecting samples of 15 grabs, i.e., trebling the sample size would have resulted in 80% of the samples having more than 90 individuals. However, 20% of the samples would have had more than 1,500 individuals and maximum numbers would be between 6,000 and 25,000 individuals. Due to the physiological effects on sorters of having to deal with these large samples, sorting and identification would be far more time consuming than three times that of the present study. Thus trebling the sample size would not be practical for a large-scale monitoring program.
Standard sampling and sorting effort was required for these samples so that the data could be used for testing a RIVPACS-type model. If standard sampling effort were not required, a fixed count method would be a better approach. For fixed counts a large sample is collected. This is then split into subsamples and a sufficient number of subsamples sorted so that 100 – 300 individuals are identified. This is the approach adopted by meiofaunologists, who regularly encounter samples with densities of fauna spread over a few orders of magnitude. The United States Environmental Protection Agency also uses this method for their Rapid Bioassessment technique of rivers (Cao et al. 1998). Due to the sparse density of macrofauna at some south-eastern Australian estuarine sites, it would be unrealistic to aim at collecting 100 individuals in all samples.
The pattern for the distribution of taxa through the samples probably accurately reflects the estuarine community structure. The estuarine communities are made up of a few widely dispersed taxa (i.e. truly estuarine taxa) and a large number of primarily marine or freshwater taxa. The marine and freshwater taxa only occur fortuitously at a few sites or are in such low densities that they are only rarely collected.
For our samples, a large amount of effort was put into sorting and identifying common species present in hundreds and thousands in some samples. Other samples were quickly enumerated because there were only 20 individuals. Many of the taxa we collected were only recorded at a few sites. When using multivariate analysis, including RIVPACS-type models, rare species contribute little to community analysis but add noise to statistical solutions (Gauch 1982). Consequently, pattern resolution would probably have been higher if more animals had been identified from the sites with low numbers of individuals. A fixed count approach would reduce the effort of sorting and identifying common animals from sites with high densities, and put greater effort into finding more individuals and presumably taxa at low density sites. To some extent, this approach would increase the numbers of sites from which taxa were recorded. However, it must be realised that low species richness and presence of many rare taxa is a basic characteristic of estuarine communities and therefore samples will always reflect this.
4.2 Abiotic Effects on Abundance and Diversity
4.2.1 Salinity
Most animals are stressed by the highly variable physicochemical environment caused by the mixing of marine and fresh water. This leads to a gradient of increasing subtidal macrobenthos species diversity from the head of an estuary to the mouth (Jones et al. 1986; Montagna & Kalke 1992). Because salinity should be related to position within the estuary, it would be expected that for samples taken from a wide geographic range, there would be a correlation between salinity and the number of taxa present in the samples. Such a relationship has been found for intertidal estuarine fauna in Tasmania (Edgar et al. 1998).
4.2.1.1 Results
Grab samples with more than 300 individuals were only collected from sites with salinities greater than 15 0/00 (Fig. 4.3a). However, most of the grab samples with low numbers of individuals were collected from sites with salinities of approximately 20 0/00 (Fig. 4.3a). Although this indicates a loose correlation between salinity and numbers of individuals, no linear relationship can be fitted to this data.
The graph of salinity versus number of taxa in grab samples (Fig. 4.3b) is characterised by samples from sites with salinities less than 15 0/00 having fewer than 15 taxa; three sites with 0 0/00 salinity having fewer than 8 taxa per sample; and the three grab samples with the highest number of taxa being collected from sites with approximately 32 0/00 salinity. These characteristics resulted in the linear regression relating salinity and number of taxa being highly significant (Spp = 4.18 + 0.37[salinity], n = 89, adjusted r2 = 0.26, p < 0.001). However, as can be seen from the plot (Fig. 4.3b), salinity is of little practical value in predicting numbers of taxa in the grab samples because for any salinity, an extremely wide range of taxa could be expected.
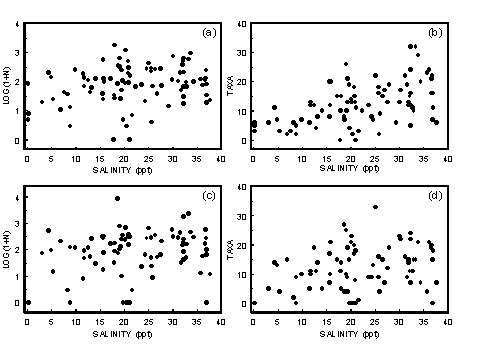
|
|
Figure 4.3 Scatterplots of the relationships between bottom salinity and a) Log(1 + N) transformed numbers of individuals in the grab samples. b) Numbers of taxa in the grab samples. c) Log(1 + N) transformed numbers of individuals in the dredge samples. d) Numbers of taxa in the dredge samples.
|
For dredge samples, there was no relationship between salinity and the numbers of individuals (Fig. 4.3c). The linear regression relating salinity and numbers of taxa in the grabs was highly significant (Spp = 6.95 + 0.24[salinity], n = 85, adjusted r2 = 0.09, p < 0.001). This is due to samples from sites with salinities less than 12 0/00 having fewer than 20 taxa and two sites with salinities less than 1 0/00 not having any fauna (Fig. 4.3d). In practical terms, this relationship has little predictive value.
4.2.1.2 Discussion
Peak numbers of individuals per sample for both methods were collected from sites with salinities of approximately 20 0/00. However, the proportion of depauperate samples was also higher at this salinity than any other so that salinity alone is a poor predictor of numbers of individuals in grab samples.
Unlike other studies, which have looked at the relationship between salinity and species richness for single estuaries, we found that when looking at data from a number of estuaries, salinity was not a very good predictor of species richness. The only relationship was maximum species richness for sites with salinities greater than 15 0/00 was higher than for sites with salinities less than 15 0/00.
4.2.2 Vertical Stratification
Approximately a quarter of the sites in this study (23 of 90) showed a marked difference (>10%) between bottom salinity and surface salinity. Usually, if stratification occurred, the upper and mid-estuarine sites were layered and the lower estuarine site if it was greater than 1.5 m deep. The majority of stratified sites were located in estuaries that were probably poorly mixed. Stratified estuaries usually had closed mouths so were not subject to tidal mixing, and they were usually narrow, which presumably prevented them from becoming mixed by wind.
The degree of stratification determines the ease with which essential gases such as oxygen can reach deep waters and support the respiratory needs of benthic communities (Costanza 1996). Layered estuaries would therefore be expected to have low densities of macrofauna and reduced species richness.
4.2.2.1 Results
The presence of a halocline can not be considered as definitely indicating poor mixing. An example of this was seen in the Snowy Estuary. The upper and mid-estuary sites were relatively shallow (<1.5 m) and at the time of sampling had an ebbing tide. The mid-estuarine site had a marked salinity difference (surface salinity 0.5 0/00 and bottom salinity 15.7 0/00) and the upper site not so marked (surface salinity 0.1 0/00 and bottom salinity 4.4 0/00). Differences at this site were probably due to fresh water streaming out over the top of the salt water on the falling tide and not due to semi-permanent poor mixing of the water at these sites.
The only azoic grab samples came from sites where there was a large difference between bottom and surface salinity (> 13 0/00) and the proportion of samples with fewer than 10 individuals increased with increasing difference between bottom and surface salinity (Fig. 4.4a). However, not all sites with a large difference between bottom and surface salinity were depauperate.
The greatest numbers of taxa in grab samples were recorded from sites where surface and bottom salinities were the same. With increasing differences between bottom and surface salinities, there was a trend for the numbers of taxa in grab samples to decrease (Fig. 4.4b).
Azoic dredge samples were more frequently collected at sites where there was a significant difference between bottom and surface salinity, but one azoic sample was recorded from a site with no difference (Fig. 4.4c). For the dredge samples, sites with a well-defined halocline either had less than 5 individuals or approximately 100. For the dredge samples there was a large proportion of samples from sites with out a halocline that had low numbers of individuals (5 to 30).
Like the grab samples, the greatest numbers of taxa in dredge samples were recorded from sites where surface and bottom salinities were the same. Also there was a trend for the numbers of taxa in dredge samples to decrease with increasing differences between bottom and surface salinities (Fig. 4.4d).
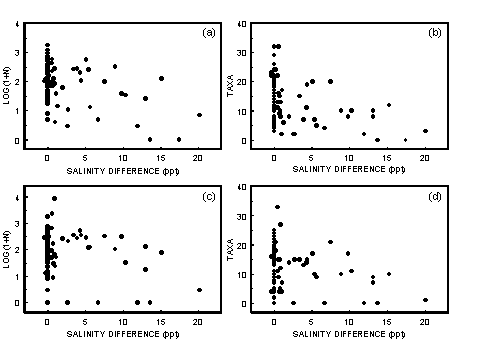
|
|
Figure 4.4 Scatterplots of the relationships for the difference between bottom and surface salinity and a) Log(1 + N) transformed numbers of individuals in the grab samples. b) Numbers of taxa in the grab samples. c) Log(1 + N) transformed numbers of individuals in the dredge samples. d) Numbers of taxa in the dredge samples.
|
4.2.2.2 Discussion
In Western Australia, it has been shown that seasonally closed estuaries undergo a daytime stratification and overnight de-stratification (Ranasinghe & Pattiaratchi 1999). In these estuaries there is very little difference between bottom and surface salinity. The differences between surface and bottom salinities in the south-eastern Australian stratified estuaries could only develop if stratification persisted over an extended period and it is unlikely these estuaries are being subjected to daily patterns of stratification and de-stratification.
Ranasinghe & Pattiaratchi (1999) revealed there are seasonal differences in mixing of Western Australian estuaries. Whether this is the case in south-eastern Australian estuaries is unknown and how this impacts on community structure and thus estuarine health assessment needs to be established.
Many samples from sites without a halocline had low numbers of individuals and low species richness, however the proportion of such samples increased for sites where there was a halocline. This suggests sites with a halocline are more likely to be environmentally stressed.
Many of the sites with a halocline were in western Victoria where most catchments have been cleared for agriculture and pastoral use. However, the area has also been subjected to comparatively recent volcanic activity, which results in the estuaries having naturally high nutrient loading. Consequently, it is not possible to state if the tendency for layered estuary sites to be depauperate is due to the shape of the estuary, high natural nutrient loadings or because of human impact on the catchments.
|
|
Figure 4.5 Scatterplots of the relationships between Log(1 + seagrass weight) and a) Log(1 + N) transformed numbers of individuals in the grab samples. b) Numbers of taxa in the grab samples. c) Log(1 + N) transformed numbers of individuals in the dredge samples. d) Numbers of taxa in the dredge samples.
|
Table 4.1 Abiotic data, abundances and species richness for the two sites sampled in the lower Tamago Estuary, one with seagrass (Site 80) the other without seagrass (Site 81). (SOC sediment organic content). |
| | SITE 80 | SITE 81 |
Depth (m) | 0.5 | 0.8 |
Seagrass (gm) | 36.2 | 0 |
Mean Particle Size (Phi) | 2.91 | 2.15 |
Sorting Coefficient | 1.03 | 0.46 |
% Silt | 7.6 | 0.4 |
% Gravel | 0.4 | 0 |
% SOC | 1.1 | 0 |
Redox | -130 | -65 |
Number in Grab | 130 | 33 |
Taxa in Grab | 21 | 11 |
Number in Dredge | 571 | 66 |
Taxa in Dredge | 21 | 18 |
4.2.3 Seagrasses
The structure of the seagrass is an important factor in determining the benthic community (Harrison & Mann 1975), and it has been shown that seagrass increases macrofaunal abundance and diversity (Castel et al. 1989).
4.2.3.1 Results
Grab samples from sites that contained seagrass had at least 33 individuals and most had between 90 and 1,000 (Fig. 4.5a). Species richness for grab samples from sites with seagrass was never below 10 taxa and the three samples with the greatest species richness came from sites with seagrass (Fig. 4.5b).
Dredge samples from sites that contained seagrass had at least 22 individuals and most had between 90 and 1,000 (Fig. 4.5c). The dredge sample with the highest species richness came from a site with seagrass and species richness for seagrass sites was not less than five taxa (Fig. 4.5d).
The lower Tamago Estuary had seagrass growing on one side and a sandy substrate on the other. This allowed the collection of samples from only 20 m apart in the same estuary, one in a seagrass bed and one from sandy sediment. The substrate at both sites was too hard for using the Ekman grab so both samples were collected with the corer, thus there was no difficulty caused by the seagrass interfering with the depth the grab penetrated and with regards to water quality the samples should be comparable. For both the grab and dredge samples, abundances were higher in the seagrass site. For the grab sample, species richness was higher in samples from the seagrass site than the sandy site. For the dredge samples, species richness was similar for the two sites (Table 4.1).
As well as the presence and absence of seagrass there were other sediment variables probably associated with changing current conditions in the seagrass bed. Sediments from the lower Tamago seagrass site (Site 80) had a smaller median particle size, a greater proportion of silt, were more poorly sorted, had a greater sediment organic content and lower redox potential than sediments from the bare sandy site (Site 81) (Table 4.1). Patterns for abundances and species richness are similar to those predicted by comparing results from seagrass samples with the other samples.
4.2.3.2 Discussion
Both dredge and grab samples indicated that relatively dense macrobenthic communities occurred in seagrass beds. Grab samples from seagrass beds were also species rich. With the exception that no dredge samples from seagrass sites were depauperate, dredge samples from seagrass beds had similar species richness to those from other habitats. The findings from the lower Tamago also fit these patterns. This indicates the presence of seagrass is an important factor that needs to be considered when assessing estuarine health based on abundance and species richness of macrobenthos.
In the present set of samples, the presence of seagrass at a site was assessed by its being in the grab samples. Because the dredge sampled a much wider area than the grabs, this method is probably unreliable for assessing if seagrass was present in a dredge site. If it were decided to sample estuarine health using only dredge samples, some reliable method for assessing presence of seagrass throughout the dredged area would be required.
4.2.4 Algae
Like seagrass, algae should provide structure allowing greater diversity of the macrobenthic community and provide an extra source of nutrients allowing greater productivity of the benthic community.
4.2.4.1 Results
Grab samples from sites that contained algae had between 38 and 1,784 individuals (Fig. 4.6a). Species richness for grab samples from sites with algae was between 8 and 18 taxa (Fig. 4.6b). Dredge samples from sites that contained algae had between 155 and 8,551 individuals (Fig. 4.6c). Species richness for dredge samples from sites with algae was between 8 and 27 taxa (Fig. 4.6c).
4.2.4.2 Discussion
Both dredge and grab samples indicated dense macrobenthic communities were associated with algal beds. Unlike the samples from seagrass beds, grab and dredge samples from algal beds were not particularly species rich.
A possible explanation for the difference in species richness patterns between algae and seagrass is that algae could occur in sites with lower salinity than seagrass. All but one site with algae occurred in the salinity range 15 to 22 0/00. Salinity for the exception was 27 0/00. Seagrasses occurred at salinities from 10 to 37 0/00. Thus, differences due to salinity appear to be unlikely.
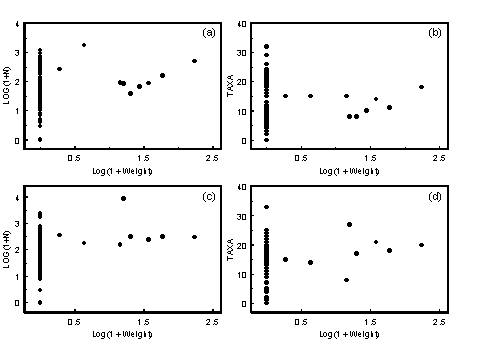
|
|
Figure 4.6 Scatterplots of the relationships between Log(1 + algal weight) and a) Log(1 + N) transformed numbers of individuals in the grab samples. b) Numbers of taxa in the grab samples. c) Log(1 + N) transformed numbers of individuals in the dredge samples. d) Numbers of taxa in the dredge samples.
|
When collecting in the field it was observed that dense, algal mats often had black anoxic sediments under them. It is possible reduced species richness for grab samples from algal sites compared to seagrass sites was due to a reduced infauna at the algal sites because of a lack of oxygen in the sediments.
4.3 Estuarine Categories
Intertidal macrofauna of Tasmanian estuaries have greater species richness in marine embayments than in estuaries exposed to a high freshwater input (Edgar et al. 1998). Edgar et al. also found species that were recorded from only one site tended to be most abundant in estuaries with a marine influence. They concluded that for the intertidal fauna there was a basic estuarine component, which was widely distributed and a component of marine species, which occurred fortuitously at a limited number of sites. If this is true for subtidal macrobenthos, it would be expected that species richness would be highest for samples from marine and classical estuaries. In addition, a greater proportion of taxa collected from only one or two sites would be expected for marine and classical estuaries.
4.3.1 Results
For the classical, marine, homogenous and layered estuarine classes several estuaries were sampled, with between 17 and 24 samples collected from each class (Table 4.2). Consequently for these estuarine classes general comparisons can be made. Only one riverine estuary was sampled (three grab sites and two dredge sites). For the riverine class, comparisons to other estuarine classes is based on averaged data from only one estuary. This is similar to pseudoreplication of samples in a badly designed experiment where only one item is being compared instead of a group of items representing the class. For this reason, sampling of additional riverine estuaries needs to be done to validate any trends indicated in the present data set for this estuary class.
Table 4.2 Information on the numbers of dredge and grab samples collected from the different estuarine categories and the numbers of individuals and taxa in the samples. |
| | Classical | Marine | Homogenous | Layered | Riverine |
G
R
A
B
| Samples | 18 | 20 | 24 | 24 | 3 |
Min N | 19 | 17 | 3 | 0 | 4 |
Max N | 1160 | 933 | 1784 | 556 | 85 |
Median N | 91 | 87 | 169 | 34 | 7 |
S Taxa | 97 | 127 | 90 | 62 | 11 |
Min Taxa | 6 | 5 | 2 | 0 | 3 |
Max Taxa | 26 | 32 | 24 | 20 | 6 |
Median Taxa | 12 | 16 | 13 | 6 | 5 |
D
R
E
D
G
E | Samples | 17 | 20 | 24 | 21 | 2 |
Min N | 9 | 0 | 7 | 0 | 0 |
Max N | 803 | 2354 | 8551 | 354 | 0 |
Median N | 117 | 166 | 177 | 95 | 0 |
S
Taxa | 80 | 141 | 91 | 70 | 0 |
Min Taxa | 4 | 0 | 4 | 0 | 0 |
Max Taxa | 25 | 24 | 33 | 21 | 0 |
Median Taxa | 9 | 15 | 14 | 10 | 0 |
For grab samples:
- Riverine and layered estuaries had high proportions of samples with low numbers of individuals (Fig. 4.7a).
- Riverine and layered estuarine samples had low median numbers of individuals (Table 4.2).
- Numbers of individuals from marine and classical estuaries were very similar (Fig. 4.7a).
- Homogenous estuaries tended to have more individuals per sample (Fig. 4.7a).
- Riverine and layered estuaries had higher proportions of samples with few taxa than samples from other estuarine classes (Fig. 4.7c). This is reflected in the low median numbers of taxa per sample from these classes of estuaries (Table 4.2).
- Marine estuaries had a high proportion of samples with large numbers of taxa (Fig. 4.7c) and a corresponding high median value (Table 4.2). The greatest numbers of taxa occurred in grab samples from marine estuaries.
- Classical estuaries had a higher proportion of taxa recorded from only a few sites when compared to the other estuarine classes (Fig. 4.7e).
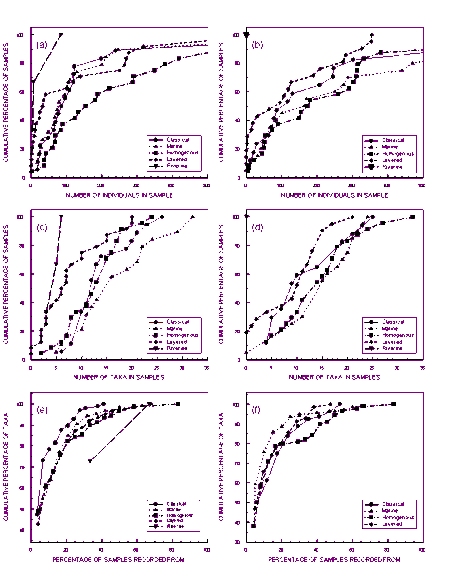
|
|
Figure 4.7 Sample profiles from the different estuarine classes. Plots of cumulative percentage of samples against the number of individuals for samples with less than 500 individuals a = grab b = dredge. Plots of cumulative percentage of samples against the number of taxa in the samples c = grab d = dredge. Plot of cumulative percentage of taxa against the percentage of samples taxa recorded from e = grab f = dredge.
|
|
(original size image)
|
For the dredge samples:
- Layered and riverine estuaries had high proportions of samples with low numbers of individuals (Fig. 4.7b).
- Classical estuaries had a higher proportion of samples with approximately 100 individuals (Fig. 4.7b).
- Homogenous estuaries had a high proportion of samples with approximately 300 individuals (Fig. 4.7b).
- Riverine and layered estuaries had a high proportion of samples with few taxa (Fig. 4.7d).
- Classical estuaries were characterised by a high proportion of samples with 5 to 10 taxa (Fig. 4.7d). Consequently, for dredge samples the median number of taxa in samples from classical estuaries (9 taxa) was less than for layered estuaries (10 taxa) (Table 4.2).
- The greatest number of taxa per sample was recorded from homogenous estuaries (Fig. 4.7d).
- For marine estuaries, there was a slightly greater proportion of taxa, which were only recorded from one or two sites (Fig. 4.7f).
For both dredge and grab samples
- The total number of taxa recorded from different estuarine classes was greatest for marine estuaries (Table 4.2).
- The total number of taxa recorded from layered estuaries was low compared with classical, marine and homogenous estuaries, considering the sampling effort was comparable for these four classes (Table 4.2).
- The proportions of taxa recorded from one or two sites were not markedly different for the different estuarine classes.
4.3.2 Discussion
Many samples from the layered estuaries were depauperate. In addition, two of the riverine sites were depauperate. However, because only one riverine estuary was sampled it is not possible to determine if this is a characteristic of riverine estuaries. It needs to be determined if these sites were naturally depauperate or if they were subjected to some unknown anthropogenic stress. If it is found that they are naturally depauperate, some way of separating naturally depauperate sites from anthropogenically stressed sites needs to be developed before large-scale regional analysis of estuarine health can be undertaken. Until there is a greater knowledge of their ecology, layered estuaries should be excluded from studies of estuarine health.
Other results are confounded by the changing efficiencies of the different collecting methods in different environments. Where the sediment was associated with complex secondary structures (e.g. seagrasses and algae), that sheltered a large epifauna community, the dredge samples had greater species richness than grab samples. Where the substrate was soft mud, which the dredge could penetrate and remove a reasonable surface layer, species richness tended to be the similar for dredge and grab samples. For harder substrates such as sand, the dredge rode over the surface without penetrating. Sandy substrates exposed to strong tidal currents had few epifauna taxa and as the dredge did not collect infauna, these sites were depauperate for dredge samples. However, because there was infauna at these sites, the grab samples contained a reasonably diverse infauna. The different substrate types are not spread randomly over the different classes. Sandy sediment without seagrasses and algae tended to be more frequently associated with areas of high tidal flow (i.e. near the mouths of classical estuaries). This is probably why 50% of dredge samples from classical estuaries had fewer than 10 taxa compared to only 10% of grab samples.
4.4 Classification of sample sites
Five different estuarine classes were recognised and four different reaches of the estuaries (bar, lower, mid, and upper), giving 20 different habitats that were to be sampled. The objective of our sampling method was to replicate sample collection at the estuary level. Replication was poorly achieved for some estuarine habitats (Table 4.3). Because the different habitats are not present in the same numbers, relying on getting a good spread of samples by selecting estuaries randomly does not work. This is exemplified by the sites in the riverine estuary where only one estuary of this class was selected for studying. For a large-scale study, this would need to be addressed in the sample design.
It is intuitive to incorporate salinity when classifying lower, mid- and upper estuarine sites. Because only classical estuaries had salinity gradients, this practice may have led to incorrectly assigning sites to the different reaches of the estuary. This was exemplified by the marine estuaries. Only two sites in marine estuaries were classified as upper estuarine. Both of these were where freshwater could be seen flowing into the estuary. Six sites in marine estuaries were classified as mid-estuarine. It is possible that upper sites in the marine estuaries where the end of the estuary could not be seen were mistakenly classified as mid-estuarine because they had high salinity.
Table 4.3 Numbers of grab samples collected from the different estuarine habitats. Where the number of dredge samples was different, these are given in brackets. |
| | Bar | Lower | Mid | Upper |
Classical | 2 | 3 | 13 (11) | 5 |
Marine | 4 | 5 | 6 | 2 |
Homogeneous | 4 | 7 | 8 | 5 |
Layered | 4(3) | 5 | 7 | 6(5) |
Riverine | 1(0) | 0 | 1 | 1 |
In the lower reaches, problems were also encountered. Due to the low tidal ranges, a large amount of sand is deposited inside the estuarine mouth (Briggs et al. 1980). This makes it difficult to separate the bar from lower reaches. For a RIVPACS model approach this does not cause any problems because these classes would not be used, they are simply a way of assuring samples were collected from diverse habitats. If a method for assessing estuarine health is to be based on comparison of communities in different habitats, then a standard method for assigning sample sites to different estuarine reaches needs to be developed.
4.5 Species Accumulation Curves
A pilot survey of four estuaries was undertaken to determine sample size requirements for the major study, a primary objective of which was to collect representative samples of the prevalent taxa at the different sites. To ascertain sample size requirements, species accumulation curves for increasing numbers of grabs were used. The intention was to estimate the number of grabs required to collect most of the taxa at a site. For this purpose, 10 replicate single grab samples were collected from each site.
The pilot survey was undertaken in summer 1996/97 (Survey 1), the large survey in autumn 1997 (Survey 2). Subsequent sets of samples were also collected in winter 1997 (Survey 3) and spring 1997 (Survey 4). Although the samples were collected in different seasons, only by showing that fluctuations in abundance and species richness are repeated over a number of years can these be proven as seasonal changes. Therefore, this set of samples only reveals temporal changes. The intention of using seasonal samples was to collect baseline data, which with future sampling may reveal seasonal patterns. The current literature based on studies of single estuaries (Jones 1987; Poore 1982) suggests south-eastern Australian estuaries are subjected to temporal changes, but not seasonal changes with peaks in abundance and species richness occurring in any season.
4.5.1 Results
The greatest numbers of individuals were collected in Survey 1, then Survey 2. Surveys 3 and 4 had similar numbers (Table 4.4). Seven of the 10 sites had the greatest median numbers of individuals in replicate grabs for Survey 1 (Table 4.5). This indicates high total numbers of individuals in Survey 1 were a general trend and not due to exceptionally high densities at a few sites. Minima were not so well defined but eight sites had the lowest or (near) equal lowest median numbers of individuals in Survey 3. This suggests the low total number recorded in Survey 4 was due to exceptionally low numbers at a limited number of sites.
Species richness as indicated by the species accumulation curves was highest for most sites (eight of the 10) in Survey 1 (Figs. 4.8 & 4.9). The two exceptions were Sites 3 and 8. Site 3 was a depauperate site during Surveys 1, 2 and 3 with the median number of individuals per grab being one or less (Table 4.5). In Survey 4, species richness and density of animals was at its highest (median of 8.5 individuals per grab). However, the total number of taxa, seven, was the same as the total number recorded in Survey 1. So although the Survey 4 species accumulation curve is higher than the Survey 1 curve, they both reach the same maximum (Fig. 4.8).
|
|
Figure 4.8 Species accumulation curves for temporal samples collected from Sites 1, 2, 3, 4, 5 and 7. Curves have been calculated using the Excel spreadsheet program Cumulate written by Karl Inne Ugland. Points are the average calculated by randomly selecting 100 permutations of the samples. Error bars are 95% confidence intervals.
|
|
(original size image)
|
|
|
Figure 4.9 Species accumulation curves for temporal samples collected from Sites 8, 10, 11, 12, and 70. Curves have been calculated using the Excel spreadsheet program Cumulate written by Karl Inne Ugland. Points are the average calculated by randomly selecting 100 permutations of the samples. Error bars are 95% confidence intervals.
|
|
(original size image)
|
Table 4.4 Total numbers of individuals for temporal samples excluding Site 70, which was not sampled in Survey 1. |
| | Survey 1 | Survey 2 | Survey 3 | Survey 4 |
Individuals | 8,977 | 3,108 | 1,779 | 1,564 |
Table 4.5 Median numbers of individuals collected per replicate grab for the different sites sampled in the temporal study. |
Site | Location | Survey 1 | Survey 2 | Survey 3 | Survey 4 |
1 | Hopkins, lower | 109.5 | 52.5 | 24 | 25.5 |
2 | Hopkins, mid | 6.5 | 3 | 3 | 4 |
3 | Hopkins, upper | 1 | 0 | 0.5 | 8.5 |
5 | Clyde, lower | 267 | 25 | 10 | 17 |
4 | Clyde, mid | 26.5 | 13.5 | 10 | 14 |
7 | Wingan, lower | 317.5 | 134.5 | 43.5 | 46.5 |
70 | Wingan, lower 2 | | 46 | 105 | 42 |
8 | Wingan, mid | 30.5 | 23 | 32 | 16 |
10 | Darby, lower | 8 | 12 | 28 | 5.5 |
11 | Darby, upper | 12 | 0 | 0.5 | 0 |
12 | Darby, mid | 3 | 2 | 0 | 1 |
This indicates the total numbers of species present at the site were similar for both surveys. However, the shape of the Survey 1 curve was restricted because there was a median of one individual per sample, with the consequence that the average species accumulation rate could not exceed one species per grab. For Site 8 the median number of individuals collected per grab in Survey 1 (30.5) was almost as high as the maximum for the site (32, in Survey 3) (Table 4.5). Consequently, the low species richness was not due to low numbers of individuals in the samples. In fact, the Survey 1 species accumulation curve is identical to the Survey 4 curve when the density of individuals was at its lowest (median per grab 16). This indicates that, unlike all the other sites, species richness was definitely low at Site 8 in Survey 1.
For the major survey, designed after reviewing the Survey 1 results, five grabs were combined to give a single sample. Generally Survey 1 had more individuals and a greater number of taxa per grab than Survey 2 (Figs 4.8 & 4.9). Five of the 10 sites sampled in Survey 1 had an average number of species in five grabs twice that of the Survey 2 samples. The largest difference was at Site 5, near the mouth of the Clyde Estuary at Batemans Bay. In Survey 1 the mean number of taxa recorded per five grabs was 54, in Survey 2 it was 19.4. Although the species accumulation curves are different for the pilot survey and the main survey, the species accumulation curves are levelling off after five grabs because there were less taxa at the sites in Survey 2 than in Survey 1.
4.5.2 Discussion
There was a large temporal difference in the numbers of individuals collected. The lowest numbers of individuals were in Surveys 3 and 4. Survey 2 had almost twice the numbers for these surveys. In Survey 1 there were five times as many individuals as in Surveys 3 and 4. Generally, the more individuals identified the greater the number of taxa recorded. As would be expected, Survey 1 samples, with the greatest numbers of individuals, usually contained more taxa per grab than the other samples. The differences in the average number of taxa per grab for Survey 2 and Surveys 3 and 4 were not as distinct as that between Survey 1 and the other surveys. There have been a number of diversity indices developed to cope with the fact that when more individuals are identified, more taxa are recorded. Such indices depend on the assumption that community structure does not change between samples. It would appear there was a change in community structure between Surveys 2 and 3 so it is not valid to use such indices to compare diversity for this set of samples.
The results clearly show there are temporal differences in densities and species richness, which are synchronised regionally. Because of this, there is a danger the results of the pilot survey may have been inappropriate for designing the major survey. Although numbers of taxa at the sites were less in Survey 2 than Survey 1 the curves were still levelling off after five grabs, even more so than for the December samples. Considering sorting times and the information gained by collecting more grabs, five grabs was also thought to be a suitable number to sort in the Survey 2 samples.
There have been two long-term studies of south-eastern Australian estuaries (Jones 1987; Poore 1982). These found temporal changes in abundances and species richness but no seasonal patterns (i.e. the same temporal patterns were not repeated over subsequent years). It might be expected that temporal patterns would differ for different estuaries (i.e. differ spatially). This was the case for macrobenthos of Tasmanian estuaries and marine embayments (Moverley & Jordan 1996).
Benthos in the subtropical Calliope Estuary at Gladstone in Queensland had distinct seasonal patterns, with density and species richness maxima in late spring or early summer and minima in winter (Moverley et al. 1986). Theoretically this pattern should exist worldwide for temperate mixed estuaries because of seasonal patterns in phytoplankton production, animal and microbial activity and mineralisation of detrital material (Levinton & Stewart 1988).
Because species richness in Survey 1 was high over such a broad geographic range, the possibility of it being a seasonal occurrence needs to be considered. The absence of seasonal patterns in the Hawkesbury (Jones 1987) and Gippsland Lakes (Poore 1982) could be because over the past 20 years, rainfall in eastern Gippsland and southern New South Wales has not been seasonal (Bureau of Meteorology, rainfall records). Thus during the studies of Jones and Poore, high rainfall could have occurred at any time and rainfall may be more influential on benthic community structure than seasonal cycles. It could also be argued that our data show there was no seasonal pattern because the Survey 1 samples were collected early in summer (12 – 20 December 1996) and the spring samples mid- to late spring (28 October – 11 November 1997). If the pattern did follow that predicted by Levinton & Stewart (1988), numbers should have been increasing when the spring samples were taken. Only at Site 3 was there evidence of species richness increasing in spring.
The temporal studies highlight that benthic community structure for a sample is not directly comparable with another collected from the same site at a different time. Because species richness and/or diversity are key components in looking for environmentally induced community changes, a drop in species richness, as observed between Survey 1 and Survey 2, can have a profound impact on environmental health assessment. Comparing the Survey 1 samples with subsequent samples would lead to the conclusion there had been a decrease in health at most sites. Before monitoring of estuarine health can be undertaken it will be necessary to develop an understanding of such temporal changes.
Before we can establish protocols for the assessment of environmental health using macrofauna, it is necessary to better understand such changes in species richness and incorporate them into any models that may be produced. If the changes are seasonal, this could be compensated for by comparing samples collected at the same time of the year or by averaging a number of samples over the year as they do in the AusRivAS model. Averaging the samples could also be done if the changes are not seasonal but maxima and minima occur over a year. However, if the patterns take a few years to change and are unpredictable, then any regional assessment of estuarine health would be difficult. It would be necessary to collect a complete set of samples each time and make comparisons based on reference estuaries. Under such conditions widespread deterioration or improvements to estuarine health would not be detected.
These results also highlight the need to collect samples for a regional assessment of environmental health over as short a period as possible. To collect the samples for the major survey took a month (10 March to 10 April) of intensive fieldwork including weekends and public holidays. Because of the high organic content of the estuarine sediments, it is impossible to buffer the formalin used to fix the samples. Consequently, the samples become acidic and the shells of molluscs dissolve adding greatly to the problems of identification. While doing the fieldwork, extra staff were hired to work at the museum washing the formalin from fixed samples and transferring them to ethanol. Without the additional staff and without working on weekends and public holidays it would be easy for the sampling of 30 or more estuaries to take several weeks. The results show that major changes could occur to species richness over such a period. An extended sampling period may result in early samples having vastly different community structures compared to latter samples. Such results would confound attempts to assess estuarine health from such a data set.
4.6 Conclusions
Estuarine samples collected by both grab and dredge were characterised by the numbers of individuals in the samples covering four orders of magnitude, most of the taxa only being recorded from one or two sites and very few taxa being recorded from many sites. Because of the wide range in the density of estuarine macrofauna, better results could probably be obtained if instead of using equal sized samples fixed counts were used to assess estuarine health.
A large proportion of the samples was collected from depauperate sites. If these are found to be natural, it presents two problems with interpreting broad scale estuarine health surveys using macrobenthic community data. Firstly, how do you assess the health of a site where only a few individuals are collected in the samples? Secondly, how do you separate naturally depauperate sites from sites that are depauperate because of anthropogenic stresses? For the grab samples, depauperate sites were associated with layered estuaries i.e. sites where there was a halocline. In the dredge samples, the proportion of azoic samples was higher for layered estuaries. Considering the difficulties of assessing the health of naturally depauperate estuarine sites it would be wise if future studies exclude or at least separate layered estuaries from the other classes.
Chapter 5: Multivariate Analysis
- 5.1 Hierarchical Clustering
- 5.1.1 Methods
- 5.1.2 Results
- 5.1.3 Discussion
- 5.2 Two-way Table
- 5.2.1 Introduction
- 5.2.2 Methods
- 5.2.3 Results
- 5.2.3.1 Grab Samples
- 5.2.3.2 Dredge Samples
- 5.2.4 Discussion
5.1 Hierarchical Clustering
Hierarchical clustering reveals those samples that share similar species composition and abundances, i.e. they have similar communities. The underlying assumption in using this analysis is that clusters exist in the data. Where the samples are evenly dispersed throughout the hyperspace described by the species composition then the clustering technique imposes an arbitrary clusters (Gauch & Whittaker 1981).
5.1.1 Methods
Hierarchical clustering was undertaken using Plymouth Marine Laboratory's PRIMER software. Samples where no fauna were collected (2 grab samples and 7 dredge samples) were excluded from the analysis. Data was double root transformed. The Bray-Curtis similarity measure was used and clusters formed by group averaging.
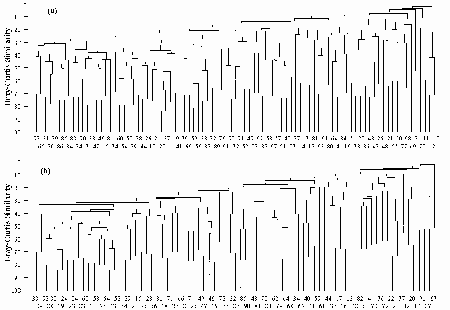
|
|
FIGURE 5.1 Dendrogram using group average clustering from Bray-Curtis similarities on ÖÖ-transformed abundances for a) the 87 grab samples b) 77 dredge samples.
|
|
(original size image)
|
5.1.2 Results
Results for the grab and dredge samples were similar. Most pairs of samples had a Bray-Curtis similarity of 40 to 60% and subsequent higher order linkages tended to occur at approximately the same levels of similarity (Fig. 5.1). This produces a pattern referred to as chaining, which indicates there are no intrinsic groups in the data.
5.1.3 Discussion
Hierarchical clustering reveals that samples are more or less evenly dispersed throughout the hyperspace described by species abundances, revealing our samples were not drawn from a limited number of discrete estuarine communities. This pattern is not unusual for ecological data (Gauch & Whittaker 1981).
There is no set level of similarity for samples drawn from similar habitats. Pairs of replicate samples drawn from the same site usually have a Bray-Curtis similarity of 70 to 80% (see examples in Clarke & Warwick 1994). The comparatively low level of similarity between the pairs of samples in these data sets (40 to 60%) indicates substantial differences between the communities from different sites.
5.2 Two-way Table
5.2.1 Introduction
PATN two-way tables summarise results of multivariate analysis and are useful for detecting the attributes responsible for groupings as well as finding any misclassifications (Belbin 1993). For species groups it is easy to see the sites where they occurred at high densities and the sites where they were absent. For site groups, the species present at high densities or absent are easily detected.
Two-way tables are also used to identify different species associations and to identify sites with unusual species associations. Common causes for unusual species associations are that the site represents a habitat that was infrequently sampled (e.g. marine embayment communities), the site represented a rare habitat or the site was stressed.
5.2.2 Methods
Two-way table analysis was undertaken using CSIRO's Division of Wildlife and Rangelands Research software PATN. Samples with no fauna (2 for grab samples, 7 for dredge samples) were excluded from the analysis. Data was log(1+n) transformed to decrease the emphasis on abundant taxa. Associations between sites were analysed with the ASO (association between rows) program using the Bray-Curtis similarity measure. Associations between taxa were also analysed using the ASO program with the two-step similarity index. The two-step index has a close affinity to the Bray-Curtis similarity measure but is optimised to find associations of attributes rather than objects (Belbin 1993). For both site and taxa cluster analysis the flexible UPGMA algorithm with a Beta value of zero was used. This gives equal weighting to the objects throughout the fusion process (Belbin 1993).
In producing the two-way table, taxa are ordered into the groups found in the ASO analysis, their values were standardised by the total number of animals in a sample, divided into five ranges (zero and four equal divisions) and a symbol given to represent the range. The standardisation by sample means the abundance of a species at a site is compared to that of the other taxa present at that site but is independent of abundance at other sites. Thus a taxon for which only 15 individuals were collected could be recorded as of little importance at a site where 10 of the individuals were collected if there were hundreds of specimens of other taxa collected from the same site. This is different from the normal two-way table analysis where standardisation is by the number of individuals in a taxon. Standardisation by individuals in a taxon was inappropriate for this data set because numbers of individuals covered four orders of magnitude, and many taxa were super-abundant at a few sites. Standardising by numbers of individuals simply revealed high proportions of individuals for most taxa occurred at sites where abundances were high.
The resulting tables were complex. Groupings were re-ordered to emphasise species groupings with low numbers of taxa present or with taxa not recorded from other sites. Some species groups were split to emphasise the distribution of coherent groups of taxa, which were only a subgroup in the original analysis.
5.2.3 Results
The resulting tables are a matrix of 87 sites by 197 taxa for the grab and 78 sites by 188 taxa for the dredge. These tables are presented in Appendix 2.
5.2.3.1 Grab Samples
The initial analysis produced a complex table with 24 site groups and 23 species groups. Several of the species groups were only collected from one or two sites and several site groups only had one or two sites. This indicates there were a large number of distinct assemblages that were only sampled once or twice.
Table 5.1 Sites, which contained species groups only collected from one or two sites and thus were probably from habitat types rarely sampled in our survey. Species groups are given in the Appendix 2 Table 1. |
| Sites | Species
Groups | Sal
(ppt) | Habitat Type |
10, 12 | 23 | > 0.3 | Both in Darby Estuary, with unique low salinity. |
77 | 12 | 37.0 | The deepest site sampled (11 m) and high salinity (37 ppt) in the mouth of a tidal New South Wales Estuary. |
13 | 22 | 32.3 | Muddy site in the head of a Victorian inlet with only a small stream flowing in to it. The stream was too shallow for us to enter and sample. Thus the location was probably a marine embayment rather than an estuary. |
14 | 21 | 31.8 | Deep site (4.5 m) with sand substrate from the middle of the same inlet as EH13. |
91 | 20 | 26.9 | A deep site (6 m) in the lower\mid-reaches of a non-tidal Victorian Estuary with slimy anoxic sediment not seen at any other site. |
80 | 14, 17 & 18 | 36.8 | Seagrass bed at the mouth of a tidal New South Wales estuary. |
05 | 15, 16 & 17 | 35.8 | Silt site at mouth of a tidal New South Wales estuary. |
76 | 15, & 19 | 36.2 | Mid-estuary site from a New South Wales tidal estuary. |
Table 5.2 Environmental conditions at sites where less than 10 taxa were collected in the grab samples. Included are the two sites that were excluded from the two-way analysis because no fauna were collected. |
Site | No.
Taxa | No.
Indivs | Depth | Sur.
Sal | Sed.
Sal | Possible reasons for depauperate fauna |
03 | 2 | 2 | 5.0 | 4.5 | 12.2 | Anoxic |
11 | 3 | 4 | 2.5 | 0.2 | 0.2 | Low salinity |
35 | 0 | 0 | 4.0 | 7.3 | 21.0 | Anoxic |
40 | 2 | 3 | 1.5 | 24.8 | 25.8 | Anoxic |
45 | 0 | 0 | 4.0 | 0.3 | 17.8 | Anoxic |
50 | 4 | 4 | 5.0 | 13.0 | 19.7 | Anoxic |
51 | 2 | 2 | 5.0 | 6.25 | 8.8 | Anoxic |
55 | 5 | 12 | 3.5 | 3.2 | 8.8 | Upper estuary |
57 | 2 | 10 | 1.0 | 4.9 | 4.9 | Unknown |
67 | 3 | 6 | 2.5 | 1.4 | 21.6 | Anoxic |
69 | 6 | 13 | 6.0 | 28.0 | 29.3 | Unknown |
78 | 6 | 22 | 1.8 | 37.8 | 37.8 | Fast flowing channel at mouth |
87 | 7 | 276 | 0.5 | 18.7 | 18.7 | Upper estuary |
There were six species groups with taxa only collected from one or two sites. These samples probably represent habitat types that were rarely encountered in the survey. Information on the sites is given in Table 5.1 along with the species groups that characterise them. Many of these sites contained high salinities, and this results probably highlights the low number of high salinity sites sampled.
There were seven single site groups and one group of four sites with very few taxa (Table 5.2). Sites 03, 11, 50, 55 and 69 belonged to single site groups that had very low numbers of individuals (<14) (Table 5.2) and the taxa present appeared to be randomly selected from the pool of widely dispersed estuarine species. Site Group 24 (Sites 40, 51, 57 and 67) also had low numbers of individuals and low numbers of taxa (Table 5.2), but they all had the polychaete Simplisetia aequisetis. These nine sites were probably severely stressed sites and should be included with the Sites 35 and 45) where no fauna was collected. Possible reasons for the sites being stressed are listed in Table 5.2.
Sites 78 and 87 belonged to single site groups that had low numbers of taxa but reasonable numbers of individuals, these sites probably represent habitat types that were only sampled once. Site 78 was the mouth of Bilba Bilba Creek, a fast flowing channel where the sediment was highly mobile sand. It was the only sample from such a habitat. Site 87 was in the upper estuary of Brou Lake. This site was characterised by few taxa but extremely high numbers of the polychaete Orthoprionospio cirriformia. O. cirriformia appears to be well adapted for living at low salinities being one of the taxa collected from the Darby Estuary where salinities were less than 1 0/00. Site 87 was probably an upper estuary assemblage that was only sampled once.
Because of the confusing number of site and species groups, only a few meaningful species groups can be recognised from the two-way table. Species group 1 had 44 taxa, which were widely dispersed and frequently the most abundant taxa. These species are the basic estuarine species adapted to a variety of estuarine conditions. Species group 2 had 45 taxa, which were widely dispersed species but infrequently collected, rarely forming a high proportion of individuals at a site. These are the rare estuarine species and marine species, which commonly enter estuaries. Species groups 8 and 9 had eight taxa, which were associated with sites where the salinity was low, or if the salinity was high, the sites were in the upper estuary. These are probably estuarine species adapted for low salinity or freshwater invaders of the estuary.
Site group 1 consisted of sites with high numbers of individuals in layered estuaries (many shallow sites), sites where the channel was hard substrate so the samples were collected on the edge and Gippsland Lakes sites. Only taxa belonging to species groups 1, 2, 3 and 8 were repeatedly collected from this site group.
5.2.3.2 Dredge Samples
The initial analysis produced a complex table with 27 site groups and 17 species groups. As with the grab samples, there was a considerable number of site groups with only one sample and species groups recorded from one or two sites.
Six site groups were differentiated by several taxa only collected from one or two sites. Information on these sites is given in Table 5.3. All but one of these sites had high salinities and all were tidal. These sites had a strong marine component in their fauna. Site group 15 (Site 33), in the upper Snowy Estuary, was the exception. It had low salinity and contained a number of insect taxa and freshwater bivalves, several of which were not collected from other sites. As well as the freshwater fauna there were seven taxa in species group 9, which contains species with dense numbers at most estuarine sites. Thus it represents a site where there was a mixing of freshwater and estuarine species. It is the only sample collected from such a habitat.
There were six single site groups, one group with two sites and one with three sites, which had very few taxa (Table 5.4). Sites 59, 50 and 67 belonged to single site groups with very low numbers of individuals (less than seven) and the taxa present appeared to be randomly selected from the total pool. These are probably highly stressed sites with sparsely dispersed fauna. Sites 03, 10, 11, 12, 35, 51 and 63 from which no fauna were collected should also be included in this group of depauperate sites. Site group 26 contained three sites (40, 65 and 41), that had only four taxa. All three of these sites had a high proportion of Simplisetia aequisetis in the sample. Single site groups for Sites 91, 87 and 20 and the two sites in site group 27 (Sites 21 and 22) had few taxa, but comparatively high numbers of individuals. Four of these were upper estuarine sites and may have had fauna specialised for these habitats. These groups probably represent communities that were infrequently sampled in the survey.
Table 5.3 Sites containing several taxa only collected from one or two locations and thus probably came from habitat types under-represented in our collection. Species groups are the groups in the Appendix 2 Table 2 that characterise the site group. S.G. is species group and Sal is the bottom salinity recorded from the site. |
Sites | S. G. | Sal | Habitat Type |
72 | 1 | 36.9 | Shallow tidal site at mouth of New South Wales lagoon |
76 | 2 | 36.2 | Mid-estuary site from a New South Wales tidal estuary. |
77 | 3 | 37.0 | The deepest site sampled (11 m) and high salinity (37 ppt) in the mouth of a tidal New South Wales estuary. |
33 | 4 | 4.4 | Shallow tidal site from the upper Snowy Estuary |
14 | 5 | 31.8 | Sandy site in the middle of a Victorian lagoon with only a small stream flowing into it. This was probably a marine embayment. |
13 | 6 & 7 | 32.3 | Shallow muddy site at the head of the same inlet as EH14. |
Table 5.4 Environmental conditions at sites where less than 10 taxa were collected in the grab samples. Included are the two sites that were excluded from the two-way analysis because no fauna was collected. |
Site | Taxa | Indivs | Depth | Sur.
Sal | Bot.
Sal | Possible reasons for depauperate fauna |
91 | 7 | 52 | 6.0 | 25.8 | 26.9 | Sediment an anoxic slime |
87 | 4 | 73 | 0.5 | 18.7 | 18.7 | Upper estuary |
59 | 5 | 7 | 0.2 | 11.5 | 11.5 | River bar, low salinity |
50 | 1 | 1 | 5.0 | 13.0 | 19.7 | Anoxic |
20 | 5 | 19 | 0.2 | 8.3 | 8.3 | River bar, low salinity |
67 | 1 | 2 | 2.5 | 1.4 | 21.6 | Anoxic |
40 | 4 | 23 | 1.5 | 24.8 | 25.8 | Upper estuary |
65 | 4 | 9 | 0.1 | 19.4 | 19.4 | Fast flowing sand bar |
41 | 4 | 8 | 2.0 | 25.3 | 26.0 | Unknown |
21 | 5 | 72 | 0.5 | 3.1 | 3.1 | Upper estuary |
22 | 5 | 29 | 1.5 | 13.3 | 14.1 | Upper estuary |
03 | 0 | 0 | 5.0 | 4.5 | 12.2 | Anoxic |
10 | 0 | 0 | 2.5 | 0.2 | 0.2 | Low salinity |
11 | 0 | 0 | 2.5 | 0.2 | 0.2 | Low salinity |
12 | 0 | 0 | 4.0 | 0.3 | 0.3 | Low salinity |
35 | 0 | 0 | 4.0 | 7.3 | 21.0 | Anoxic |
51 | 0 | 0 | 5.0 | 6.3 | 8.8 | Anoxic |
63 | 0 | 0 | 1.0 | 37.0 | 37.0 | Unknown |
As with the grab samples, the dredge two-way table identifies a species group (Group 9, which had 47 taxa) that were widely dispersed, frequently collected, and in high abundance at many sites (i.e. common estuarine taxa). Another group (Group 8, which had 28 taxa) had taxa that were widely dispersed, but infrequently collected and infrequently highly abundant at a site (i.e. rare estuarine taxa and marine taxa frequently encountered in the estuary). No species group associated with several low salinity sites could be identified in the dredge samples.
5.2.4 Discussion
The two-way table identifies 19 of the grab samples (21 when the two without fauna are included) and 24 (31 when the seven without fauna are included), which contain species assemblages found at less than five sites. Because these species assemblages are not well represented, they are poorly resolved in the multivariate analysis but add greatly to the complexity of the analysis. Because a high proportion of the samples represent communities that are not well represented in our samples, or sites where sampling was insufficient to adequately describe the community present, difficulties could be expected in trying to develop community based estuarine health models from this data set. These results suggest that future sampling should address this deficiency by targeting additional samples from unusual environments.
Chapter 6: RIVPACS Model Approach
- 6.1 Methods
- 6.1.1 Environmental Data
- 6.1.2 Faunal Data
- 6.1.3 Model Construction
- 6.1.4 Test Sites
- 6.1.5 Data Matrix
- 6.2 Results
- 6.2.1 Discriminant Analysis
- 6.2.2 Testing
- 6.2.3 Family Level Identifications
- 6.3 Discussion
RIVPACS (River Invertebrate Prediction and Classification System) models were developed in the early 1980s to assess river health in the United Kingdom (Moss et al. 1987). As well as the United Kingdom, RIVPACS-type models (AusRivAS) are now being used throughout Australia for river health assessment (Marchant et al. 1997; Parsons & Norris 1996). With the exception of some application within lakes (Johnson & Wiederholm 1989; Reynoldson et al. 1995) these models are only just starting to be applied to non-river ecosystems.
RIVPACS-type models produce a list of taxa and their probability of being collected from a site. The number of species expected to be in a sample can be calculated from the probabilities. The ratio of the observed taxa to expected taxa (O/E) is then used as an index to environmental health. A value of one indicates the expected community is present. A value that deviates from one, in either direction, indicates some disturbance has changed the community.
Random sample variation means some deviation from one will occur even at pristine sites. Where the expected number of taxa is large, a difference of one or two taxa is comparatively minor (e.g. with an expected value of 20 taxa, missing one due to random variation gives an O/E of 0.95). However, where the expected number of taxa is small, a difference of one or two taxa causes substantial differences (e.g. with an expected value of 4 taxa, one missing due to random variation gives an O/E of 0.75). Consequently, RIVPACS-type models are not very practical for monitoring environmental health where there are few taxa with a high probability of being collected in the samples.
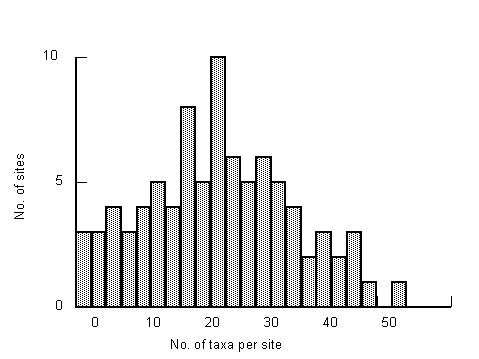
|
|
Figure 6.1 Histogram showing the number of taxa recorded per site for the combined grab and dredge data set (85 sites).
|
The univariate analysis (Chapter 4) revealed many of our samples had few taxa and large proportions of the species were only collected from a limited number of sites. As would be expected from such data, preliminary modelling of the grab and dredge data sets revealed inadequate numbers of taxa per site group for RIVPACS-type models to be of value in environmental health monitoring.
In order to increase the number of taxa recorded per site, the grab and dredge samples were combined. Providing the same sampling methods are used at each site it is possible to construct a RIVPACS-type model for that sampling method, or combination of methods. The mean number of taxa recorded per site for individual grab and dredge samples were approximately 12. For combined grab and dredge samples, the mean number of taxa per sample was 19.45. However, like the grab and dredge sample profiles, the number of taxa in combined grab and dredge samples still varied considerably (Fig. 6.1).
6.1 Methods
6.1.1 Environmental Data
The environmental variables (Table 3.1) were checked for normality using the normal distribution probability plot routine in SYSTAT, and where necessary were transformed using log (x + 1) or arcsine transformations.
Complete data existed for all variables with the exception of redox (five missing values), sediment data (clay, gravel, silt, sand, organic carbon, mean particle size and interstitial salinity) (two missing values) and bottom and surface salinity (one value missing). Where possible missing values were substituted using regression analysis.
6.1.2 Faunal Data
Only taxa collected from more than three sites were used in the model construction. Faunal data was transformed to presence/absence data. Two sets of data were used. The first was with species level identifications. This data set was used for the full development and testing of the model. The second set used family level identifications. This data was only worked up to the stage where it could be shown that the expected number of taxa in the samples was similar to that of the species level identification. Therefore, the family level model would not be more robust than the model using species level identifications.
6.1.3 Model Construction
The following steps described in Moss et al. (1987) and Marchant et al. (1997) were used to produce and test the predictive models for the grab and dredge samples separately and then for the combined grab and dredge samples.
- Reference sites were placed into groups using cluster analysis (UPGMA or TWINSPAN).
- Predictive environmental variables were chosen using stepwise Multiple Discriminant Function Analysis (MDFA). This procedure utilises a subset of the environmental variables to distinguish the site groups along a number of linear axes (the discriminant functions) that describe variation in the environmental data.
- The probabilities for a test site belonging to each of the site groups were calculated from the discriminant model.
- The probability of occurrence of a given taxon at a site was calculated by multiplying the outcome of step 3 by the percentage frequency with which the taxon occurred in each site group.
- For each taxon, the probabilities of it being in each of the site groups were summed to give an overall (or weighted average) probability of occurrence.
- The taxa with > 50% overall probabilities of occurrence are identified - these are the predicted taxa.
- The number of expected taxa at the site equals the sum of the individual probabilities of occurrence of all the predicted taxa. (Hence, the number of expected taxa is always lower than the number of predicted taxa.)
- Observed taxa are the predicted taxa found in the sample. Therefore, the number of observed taxa is not the total number of taxa in the sample but the number of predicted taxa.
- The number of observed taxa was divided by the number of expected taxa to give an O/E ratio. The closer this ratio is to 1.0 the closer the observed community conforms to expectation.
A program written in SAS (Statistical software) by the Cooperative Research Centre for Freshwater Ecology (University of Canberra) was used to perform these procedures.
A dissimilarity value of 0.80 was used as a guide when attempting to place the sites into groups using UPGMA cluster analysis. This was the value used for the Australian AusRivAS predictive models (Simpson et al. 1996). Linkages at higher levels of dissimilarity indicate sites with few taxa in common. Such groups would have very few predicted taxa because there would be a very low number of taxa with a 0.5 probability of being collected.
Table 6.1 Ranked position along the coast, site no., surface salinity (0/00) and bottom salinity (0/00) Depth (m) for the seven test sites. |
Site
No. | Ranked
Position | Surface
Salinity | Bottom
Salinity | Depth |
53 | 12 | 5.7 | 15.5 | 2.2 |
40 | 24 | 24.8 | 25.8 | 2.2 |
18 | 36 | 10.1 | 19.0 | 1.5 |
29 | 48 | 20.9 | 21.1 | 2.0 |
07 | 60 | 32.8 | 33.4 | 2.0 |
72 | 72 | 36.9 | 36.9 | 0.2 |
81 | 86 | 37.1 | 37.1 | 0.8 |
Only taxa with a probability of 0.5 or greater have been used as expected taxa. RIVPACS analysis has traditionally only considered those taxa predicted to occur at a site with a probability of 0.5 or greater. Although this is an arbitrary cut-off, it excludes those taxa, which occur so infrequently at reference sites that predictions of their occurrence are unreliable. In addition, taxa with less than a 50% probability of occurrence contribute little to the number of expected taxa (Simpson et al. 1996). This is because the level of probability tails off quickly, with most samples made up of taxa with relatively high probability of being collected and a number of taxa with very low probability of occurrence (i.e. rare taxa). Many taxa with a low probability of occurrence are required to increase the expected taxa by one, e.g. five taxa with a probability of 0.2. Consequently, even when taxa with less than 0.5 probability are included there are only small increases in the numbers of expected individuals.
6.1.4 Test Sites
The predictive capacity of the model was tested by applying it to a number of test samples not used in the model construction. Test sites needed to be representative of conditions throughout the region sampled. As geographic variation (east-west) was found to be an important factor in explaining community patterns in the data, position along the coastline was used to stratify the sites. Every twelfth site along the coast (i.e. seven of the sites) was selected as a test site and not used in the modelling (ie. group classification, MDA, predictions etc.). These sites contained a range of salinities and depths (Table 6.1).
Site 40 was unsuitable as a test site due to the low number of taxa recorded (two in the grab sample and four in the dredge sample). Consequently, only six test sites were used to evaluate the model.
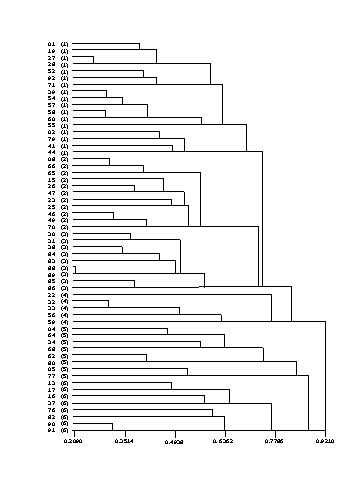
|
|
Figure 6.2 UPGMA dendrogram for the combined data showing the site groups based on species level identifications used in the predictive model (groups 1 – 6).
|
6.1.5 Data Matrix
There were 84 sites where both grab and dredge samples were collected. Some of these sites could not be used for a RIVPACS-type model because they had inadequate environmental data others were not used because they only had one taxa, which was considered to be too few for model construction. The initial data matrix had 71 sites by 80 taxa.
At a dissimilarity value of 0.80, 12 outlying sites did not fit into any groups. As well as having few shared taxa, these sites had low numbers of taxa (three to seven). They were considered unsuitable as reference sites in a predictive model. These twelve sites and one other site with only four taxa were omitted from further modelling, leaving the remaining sites with between eight and 32 taxa. The dimensions of the final data matrix used to produce the model were 58 sites by 76 taxa.
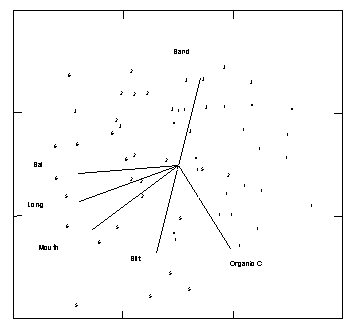
|
|
Figure 6.3. Two axes of a three dimensional MDS ordination of the 58 combined reference sites showing the position of the UPGMA groups and the direction of the PCC correlation vectors for selected variables (stress = 0.24).
|
6.2 Results
Six site groups (Fig. 6.2) were selected from the UPGMA cluster analysis and MDS ordination (Fig. 6.3). In the ordination all but group 4 are distinct (Note only two dimensions of a three dimensional plot are shown in Fig 6.2), group 4 having its sites scattered among the other groups in the three dimensional ordination.
The PCC (Principal Axis Correlation routine in PATN) correlation vectors indicate that sites in groups 2, 5 and 6 have higher salinities (r > 0.60) and were more easterly i.e. had higher longitudes (r = 0.61) than sites from groups 1 and 3 where the mouth of the estuary is also more likely to be closed (r = 0.55). Sites in groups 2 and 3 tend to have sediments with a higher percentage of sand (r = 0.41) than those in group 5. All variables quoted and displayed on the ordination plot were significantly correlated with the ordination pattern (MCAO routine in PATN).
6.2.1 Discriminant Analysis
Discriminant analysis selects a number of linear axes for environmental variables maximising the separation between site groups, while minimising the distance between sites within each group. The number of axes, or discriminant functions as they are referred to, is generally equal to one less than the number of groups or variables used in the analysis (whichever is fewer). The functions display ever-decreasing separation between the groups, with most of the separation evident in the first or second functions. Although discriminant analysis can be performed using the whole environmental variable list, a stepwise procedure is generally preferred. Because many variables have a high degree of correlation, a solution similar to that produced using all the variables, can be achieved with a substantially reduced number of variables. This is achieved in the stepwise procedure by sequentially entering the variables and selecting those that provide the best separation into the model until the remaining variables do not significantly increase separation of the groups.
Table 6.2 The nine variables that provided the best predictive power for the discriminant model. |
Variable | Transformation |
Latitude | Nil |
Water temperature | Nil |
Bottom salinity | Nil |
Mouth | Open or closed |
Catchment area | Log |
Estuary shape | Log |
% organic carbon | Arcsine |
Depth | Log |
Coastal site position | Ranked |
Table 6.3 Standardised discriminant function coefficients (1 -5) for the environmental variables used in the combined data discriminant analysis. Variables are in descending order of selection. |
Variable | 1 | 2 | 3 | 4 | 5 |
Latitude | 0.539 | 0.513 | 0.784 | 0.464 | 0.272 |
Water Temperature | -0.133 | -0.741 | -0.547 | -0.472 | 0.108 |
Bottom Salinity | -0.031 | -0.396 | 0.706 | -0.488 | 0.364 |
Mouth | 0.769 | -0.237 | 0.181 | 0.442 | -0.045 |
Catchment area | 0.709 | -0.314 | -0.154 | -0.690 | -0.217 |
Estuary shape | -0.257 | -0.433 | -0.369 | 0.575 | 0.506 |
Organic carbon | -0.014 | -0.426 | -0.091 | -0.683 | 0.402 |
Depth | -0.706 | 0.431 | 0.424 | 0.123 | -0.177 |
Coastal rank position | -0.134 | 0.595 | 1.009 | 0.539 | -0.691 |
% of dispersion | 50.1 | 23.6 | 15.1 | 7.7 | 3.5 |
The predictive power of a discriminant model can be assessed by reclassifying the reference sites using the discriminant functions. The success of correctly assigning sites to their group gives an indication of how well the variables effectively distinguish the groups. The reclassification error can then be used as a guide in selecting the optimum discriminant model/variables used (J. Simpson, University of Canberra, pers. comm.). The lowest misclassification error rate for our six groups was achieved using the variables listed in Table 6.2.
When used to reclassify the reference sites, these variables successfully classified 63% of the sites when subjected to a jackknife (cross-validation) reclassification. Jackknife reclassification is a procedure that derives functions for all sites except the site being classified. The classification matrix produced indicates that sites in group 3 were the most successfully classified (89% of sites), while sites in groups 1 and 5 were the least successfully reclassified (50 – 53%).
The standardised discriminant function coefficients for each of these variables are given in Table 6.3. The variables most strongly associated with the first discriminant function (50% of the dispersion explained) were estuary mouth (open or closed), catchment area, depth and latitude. Water temperature, coastal site position and latitude explain most of the variation on the second function. The remaining three functions only explain another 26% of the total separation between the groups (Table 6.3). The important variables in distinguishing our groups tend to be those describing geographic position and estuary morphology.
To be included in a list of expected taxa for a test site, a species must have a probability of being collected greater than 0.5 i.e. it must occur in more than half the samples of a group. Theoretically, our model predicts between seven and 18 taxa per test site (Table 6.4).
6.2.2 Testing
The combined model was used to predict community assemblages at the six test sites, the results of which are presented in Appendix 2. Four sites recorded O/E scores greater than 0.80 (accurate prediction of macrobenthic assemblage = 1.0), while the other two sites had O/E scores between 0.70 and 0.80 (Table 6.5). As expected, the number of predicted taxa varied between seven and 18, and, expected taxa varied between 4.01 and 13.08. Expected taxa number less than predicted taxa because the probability of a taxon being collected is less than one.
Table 6.4 The number of sites within each group and the number of taxa which occurred at more than 50% of those sites for the species level model.
Group | Sites | Taxa |
1 | 17 | 13 |
2 | 11 | 17 |
3 | 9 | 18 |
4 | 5 | 7 |
5 | 8 | 11 |
6 | 8 | 12 |
Where a low number of taxa are expected, a lower number of taxa are required to meet the criteria of what is expected (O/E close to 1.0). However, they must be selected from a narrower band of taxa (ie. the predicted taxa), which is also low. For example, only five of the seven predicted taxa were required for Site 7 to achieve an O/E score of 1.11. With such low numbers of expected taxa, the O/E ratio is very sensitive to sampling errors. With one fewer observed taxon the O/E score drops to 0.89, and with two fewer observed taxa it falls to 0.67. When the expected number of taxa is higher, say around 15, the effect of missed taxa is reduced and the score is likely to be more stable. The concern with this model is that for at least four of our site groupings the expected number of taxa will be below 10. Thus, it is highly susceptible to error due to random variability so that only large changes can be detected. Because of this, the model is not very sensitive.
Table 6.5 Predictive model results for each of the test sites. Abbreviations are as follows. Pred50 : No. taxa predicted, Exp50 : No. taxa expected, Obs50 : No. taxa captured, O/E50 : No. captured / no. expected. |
SITE | Pred50 | Obs50 | Exp50 | 0/E50 |
7 | 7 | 5 | 4.49 | 1.11 |
18 | 11 | 6 | 6.45 | 0.93 |
29 | 18 | 14 | 13.08 | 1.07 |
53 | 11 | 9 | 7.35 | 1.22 |
72 | 11 | 5 | 6.83 | 0.73 |
81 | 7 | 3 | 4.01 | 0.75 |
The abiotic variables are used to predicted the probability of where in the multidimensional space the test site will fall (i.e. the probabilities of the test site belonging to the different site groups). Because the multidimensional space is defined by the different taxa this information can be used to predict the probability for taxa occurring in the test site sample. The predicted taxa for a site depends on the site's position in the defined by the species that. Table 6.6 displays the group probabilities calculated by the discriminant model for the test sites. As explained earlier, the discriminant model has an error rate of 37% when assigning reference sites to site groups. Therefore, some of the test sites may be incorrectly classified. Cross validation error rates for models constructed from one set of riverine samples in Victoria with a comparable number of sites (49) had error rates far lower than this (< 15%; Marchant and Hirst unpublished data). In general, the cross validation error rates for discriminant models based on the AusRivAS monitoring river health sites (> 100 sites) are rarely higher than about 25%, although this depends to some extent on the number of groups. Inevitably, the reclassification error rate will increase as the number of groups increase. Simpson et al. (1996) argue that the actual value of the misclassification error is not critical because the cross validation procedure only allocates sites to a single group, whereas the model predictions are based on calculations using all site groups. Some discriminant models nevertheless will be better than others. These results suggest there is a lower overall correlation between habitat variables and estuarine community patterns than their river counterparts. Hence, in general estuarine models may be less accurate.
Table 6.6 Group probabilities for each of the test sites. |
SITE | 1 | 2 | 3 | 4 | 5 | 6 |
7 | 0.02 | 0.06 | 0.00 | 0.00 | 0.12 | 0.80 |
18 | 0.61 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 |
29 | 0.03 | 0.00 | 0.96 | 0.00 | 0.01 | 0.00 |
53 | 0.87 | 0.00 | 0.01 | 0.04 | 0.00 | 0.07 |
72 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.97 |
81 | 0.02 | 0.00 | 0.00 | 0.00 | 0.62 | 0.35 |
6.2.3 Family Level Identifications
The AusRivAS Model used for the assessment of river health is based on family level identifications (Davies 1994). A model using family level identifications was developed to the point of finding site groups and establishing the numbers of taxa which occurred at more than 50% of the sites in the groups. As was seen with the species level identifications, this gives a guide to the number of predicted and expected taxa a RIVPACS-type model would calculate for test sites. Thus at this stage of development we could see if a family level model would increase the numbers of predicted taxa. Increases in predicted taxa could be expected if several species, which were too infrequently recorded to be used in the species level model, were lumped together and thus reached a frequency where they were used. Some loss of expected taxa would also occur with family level identification because several families (Corophiidae, Nereidae, Hydrobiidae, Nassariidae, etc) had more than one species which were recorded frequently enough to be predicted taxa.
As with the species level identification, only taxa recorded from at least three sites were included. To keep the results directly comparable with the species level model, the same 58 sites were used. This gave a data matrix for the family level identifications of 58 sites by 69 taxa. Four site groups (Fig. 6.4) were selected from the UPGMA cluster analysis. The numbers of taxa in more than 50% of the samples within these groups (i.e. taxa with greater than 0.5 probability of being collected for the site groups) is between 10 and 19 (Table 6.7). This is only a marginal improvement on the 7 to 18 taxa found in the species level identifications (Table 6.4). Consequently, the numbers of predicted and expected taxa for a family level model would be very similar to that of the species level model. As the species level model revealed it was feasible to construct a predictive model of south-eastern Australian estuaries, it was not considered worthwhile to proceed any further with development of the family level model.
Table 6.7 Number of sites within each group and the number of taxa which occurred at more than 50% of those sites for the family level model.
Group | Sites | Taxa |
1 | 17 | 10 |
2 | 15 | 19 |
3 | 16 | 17 |
4 | 10 | 10 |
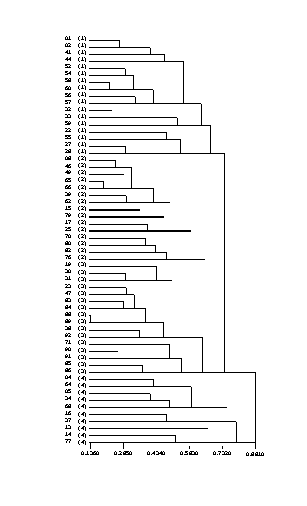
|
|
Figure 6.4 UPGMA dendrogram for the combined data showing the site groups based on family level identifications used in the predictive model (groups 1 – 6).
|
6.3 Discussion
The primary object of this project was to ascertain if RIVPACS-type models could be constructed for Australian estuaries. Predictions about estuarine benthic communities for test sites were relatively accurate (O/E scores > 0.70) demonstrating RIVPACS-type models can be constructed for south-eastern Australian estuaries and thus presumably for all Australian estuaries. Our model, intended only to test if RIVPACS-type models could be constructed for Australian estuaries, had two major weaknesses. There was a low number of reference sites and it is possible important taxa were excluded from the model's development.
The present model was constructed with very few reference sites (58) for a RIVPACS-type model. Typically, working models incorporate at least several hundred reference sites. There were two major reasons for this. Firstly, it was only a pilot survey to test the feasibility of developing an Australian estuarine RIVPACS-type model. Presumably, if a working model was to be developed, more resources would be expended so that it would incorporate a greater number of sites. Secondly, a large number of the sites sampled for this study could not be used in the model because they were depauperate. This problem could be reduced in future modelling by excluding layered estuaries from the samples or perhaps sampling at a different time of the year. The temporal study suggests that in spring and early summer layered estuarine sites may not be depauperate and therefore if sampled at this time of the year could be incorporated into a model.
Our model was constructed using only taxa that occurred in more than three samples. Taxa occurring less frequently than this will not have a 0.5 probability of occurring in site groups formed by the model, and are so infrequent that little can be inferred about their relationship with abiotic variables. In fact rare taxa may confound the results. The two-way tables (Section 5.2) revealed there were many site assemblages in our data differentiated by taxa collected at less than three sites. Many of these site assemblages were at the mouths of open estuaries or the heads of classical estuaries. These habitats were infrequently sampled in our study due to our selecting estuaries randomly and open estuaries, particularly classical estuaries, being relatively rare in south-eastern Australia. Removing these taxa from the analysis would have made these site communities more like the average sample (i.e. a mid-estuarine site) reducing the impact of the salinity gradient and decreasing the predictive power of our model. Future work could address this problem by targeting these sites and thus increasing the occurrence of taxa that may be representative of upper and lower reaches of estuaries.
Although RIVPACS-type models can be constructed for south-eastern Australian estuaries, whether they are of use for estuarine health monitoring also needs to be assessed. There are three major problems with using RIVPACS-type models for assessing estuarine health. These are choice of reference sites, low numbers of taxa and unknown impacts of temporal changes in the estuarine benthic community.
For the British RIVPACS and Australian AusRivAS monitoring programs, reference sites are located in national parks and other sites where there are no anthropogenic impacts on the habitat. Thus, the reference sites are presumed to be pristine. No such sites exist for south-eastern Australian estuaries. Rivers with the estuary and catchment in undeveloped areas are limited in geographic distribution and not representative of the full spectrum of estuaries. Even in estuaries that are in national parks, fishing and bait collection are allowed. Presumably, the removal of the upper level predators by fishing and the selective removal of species and habitat damage caused by bait collecting have an impact on the benthos. Thus, any estuarine model can not be based on pristine sites but rather on the average site as they presently are. One of the advantages in using a RIVPACS-type model is that it has the ability to detect impacts, such as mild organic enrichment, where the species diversity is increased. Such conditions result in an O/E ratio greater than one. Such results in an estuarine model could be argued to be due to the test site being healthier than the reference sites.
The temporal study highlighted there were temporal differences in numbers of individuals and taxa for site samples. This indicates the probability of a taxon being in a sample can vary with time. The literature (Jones 1987, Poore 1982) suggests these changes are not seasonal and are unpredictable. As these researchers have only looked at single estuaries, the lack of seasonal trends on a regional basis needs to be confirmed. If the changes are unpredictable, then a complete set of reference sites would need to be collected every time an assessment of estuarine health was required. It is possible that if the standard sampling technique was to examine a set number of individuals from each site, as was suggested to cope with the large range of individuals encountered at the reference sites, this problem could be reduced. If the changes are found to be seasonal then sampling could be carried out at a time when numbers of individuals and species richness are at their highest.
Our model based on combined grab and dredge samples collected only at one time predicts between seven and 18 taxa for test sites. In contrast, AusRivAS models at the family level based on one set of samples for Victorian rivers have between 12 and 24 predicted taxa (L. Metzelling, Victorian EPA, pers. com.). Rivers in the south-western region of Western Australia have low numbers of predicted taxa, which are comparable to the numbers in our study (M. Smith, WA Catchment and Land Management, pers. com.). The usefulness of the AusRivAS models in south-western Western Australia is presently being assessed.
There are numerous ways the numbers of taxa could be increased. The easiest would be to collect larger samples. Alternatively, as in the AusRivAS and the British RIVPACS programs, collect multiple sets of samples at different times (usually three, one in autumn, summer and spring) for each site. Sampling at different times of the year is important in the freshwater environment because of the large numbers of insects that may only be in the benthic community for part of the year. Collecting multiple sets of samples from estuaries would present difficulties with incorporating variables such as salinity, which undergo temporal changes, into the model. Variables used in the freshwater models are geographic and geological ones. At present salinity is important in our estuarine model because it separates samples from the mouth and head of the estuary. Some factor that does not fluctuate temporally (e.g. proportion of distance along estuary), would need to be introduced to replace this. We did not have enough data from classical estuaries to test the validity of this variable.
Our findings reveal a RIVPACS-type model can be constructed for south-eastern Australian estuaries and presumably for all Australian estuaries. For south-eastern Australian estuaries, the numbers of predicted taxa are probably at the lower limits for the successful application of such a model to environmental health monitoring. Models for estuaries in other parts of Australia where there would be greater tidal ranges and the estuaries would normally be open, could have greater numbers of predicted taxa. Consequently, we feel with further development RIVPACS models could be applied to monitoring of estuarine health in Australia.
Chapter 7: Alternative Methods
- 7.1 Multivariate Analysis of Abundance Data
- 7.1.1 Methods
- 7.1.2 Results
- 7.1.3 Discussion
- 7.2 K-dominance Curves
- 7.2.1 Methods
- 7.2.2 Results
- 7.2.3 Discussion
- 7.2.4 Testing Improving Estuarine Health, Tasmania
- 7.2.4.1 Results
- 7.2.4.2 Discussion
- 7.2.5 Testing Temporal Patterns – South-eastern Australia
- 7.2.5.1 Methods
- 7.2.5.2 Results
- 7.2.5.3 Discussion
- 7.2.6 Testing Temporal Patterns – Central Queensland
- 7.2.6.1 Methods
- 7.2.6.2 Results
- 7.2.6.3 Discussion
- 7.2.7 Assessment of k-dominance curves
Perturbation of the environment alters species abundance patterns. A number of univariate, multivariate and graphical methods have been devised to examine benthic community and indicate whether, or not, species abundance patterns are "normal". To be of value, these methods need to be sensitive enough to indicate a change has occurred, before it is obvious to the casual viewer that there has been some environmental catastrophe. This sensitivity is dependent on whether differences caused by perturbations can be distinguished from natural variation.
The data set collected to build the RIVPACS-TYPE model provides us with a data set that can be used to test and evaluate different methods for assessing environmental health based on samples collected from a wide range of geographic locations.
7.1 Multivariate Analysis of Abundance Data
Multivariate analysis techniques treat each species as a variable, and therefore are able to detect subtle differences between two communities (Norris & Georges 1993). For analysis of disturbances at point sources where communities that are closely spaced are being compared, multivariate analysis by ordination or cluster analysis has proven a useful technique to identify disturbed and undisturbed sites (Growns et al. 1995). When comparing samples from rivers where the sites have been widely dispersed, ordination frequently reveals a gradient from disturbed to undisturbed samples. However, in some environments there may be several axes that give greater separation of the samples than the axis that correlates to the disturbance (Cao et al. 1996). On a regional basis ordination has not been used to separate healthy and unhealthy estuarine sites.
In this section we investigate the potential to use multivariate analysis of abundance data to assess the health of regional estuarine sites from which grab and dredge samples were collected.
7.1.1 Methods
Abundance data for the grab and dredge samples were analysed using the Cluster and MDS programs in the PRIMER multivariate analysis package.
No samples were collected from known polluted sites. However, data for sites that were naturally depauperate, have been included. These should mimic unhealthy sites. Sites where no fauna were collected have zero similarity to all the other sites. Methods, such as those in PRIMER, that depend on comparing similarity or dissimilarity between samples are not able to incorporate these samples. Consequently, samples where there were no animals have been excluded from the analysis.
7.1.2 Results
Hierarchical clustering of the dredge and the grab samples were presented in Chapter 5 (Figure 5.1). They revealed most pairs of samples had a similarity of 40 to 60%. The dendrograms showed a high level of chaining indicating that there were no intrinsic groups in the data.
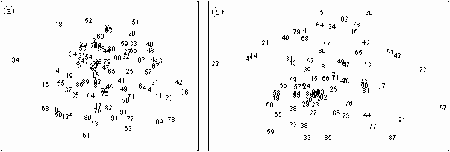
|
|
Figure 7.1 MDS plots for a) the 87 grab samples, stress = 0.27 and b) 77 dredge samples, stress = 0.23. Samples without any fauna have been excluded from analysis.
|
|
(original size image)
|
The ordination plots (Fig. 7.1) reveal that there is a cluster of sites with others radiating out from this. (Note the high stress values indicating the configuration is poorly represented by the two dimensional plot.) The actual dispersal of the samples in a multidimensional space would be the equivalent of a sphere. Because the species in the depauperate samples appear to be random subsets of the common estuarine species the samples from the depauperate sites are located anywhere on the outer margins of this multidimensional sphere.
7.1.3 Discussion
The Bray-Curtis similarity index, used in these calculations, does not consider absences. When using such an index, depauperate samples that do not have common taxa are more similar to the average sample than to each other. This occurs with many of the depauperate estuarine samples so that they are scattered around the edge of the multidimensional cluster of samples. Thus ordination does not reveal a grouping of depauperate samples.
For non-depauperate samples species composition is very diverse. This results in many of the species axes, which separate the samples in the multivariate analysis being of similar importance. Consequently, there are no readily recognised groupings in the samples. Thus minor community changes occurring as the environmental health of a site deteriorates will be impossible to detect.
Multivariate analysis could be successfully used to detect changes that stimulate certain key species to develop in disturbed areas (e.g. Capitella capitata in areas with high organic input). However, for analysis of regional data, such changes will only be detected after there have been major changes to the community. At this stage the changes would probably be obvious to the casual observer. Consequently, use of multivariate analysis would not be a practical method for monitoring environmental health.
7.2 K-dominance Curves
K-dominance curves are the abundance curves used in the ABC method of environmental health assessment. They are plots of cumulative percentage abundance versus increasing species ranked by decreasing number of individuals. This gives a graphic representation of the total number of species present (species richness) and how evenly the individuals are apportioned among the species (evenness). These are the two components of diversity (Platt et. al. 1984) so k-dominance curves allow the visual comparison of diversity for different samples.
K-dominance curves allow the ready detection of changes in samples where changes in richness and evenness produce ambiguous results in univariate indices. Examples of these situations are where richness decreases and evenness increases or richness increases and evenness decreases. Decreasing species richness and increasing evenness occurs in the meiofauna community around fish farm cages (Moverley 1995). Close to the cage, at the higher end of an increasing gradient of organic enrichment, there are extremely high densities of a few taxa, and the overall numbers of taxa decrease with increasing organic enrichment. Moving along the gradient from the optimal conditions for one species to optimal conditions for a different species, the first species decreases while the second increases. Evenness increases until both species are present in about the same number. Because evenness in such a community approaches the maximum, univariate diversity indices suggest a healthy environment. Richness can increase and evenness decrease in the transection zone between mild enrichment, where the extra food source stimulates species richness, and the highly enriched area where abundances of a limited number of taxa are high.
The structures of estuarine benthic communities resemble those in highly enriched sediments in that there are frequently a few taxa that are highly abundant. As k-dominance curves are useful for detecting changes in communities where there is organic enrichment they should also be useful for detecting changes in estuarine communities.
By plotting multiple k-dominance curves together, species abundance patterns from different assemblages can be readily compared. Such comparisons become confusing when there are more than several plots. For k-dominance curves to be of value in assessing environmental health there needs to be some method where hundreds of curves can be compared and grouped into those collected from healthy and unhealthy environments.
Multivariate analysis provides a means for sorting and grouping hundreds of objects that can be described by several different variables. In order to allow the comparison of a large number of k-dominance curves, a method of tabulating several simple community indices along with a number of values read from a sample's k-dominance curve was used. Ordination of this data is then undertaken to reveal the similarity between different samples based mainly on the features of their k-dominance curves. Samples from test sites can be analysed along with a large number of reference samples to see how the test sites compare to "typical" estuarine samples. Different areas of the ordination plot can be identified where the samples are indicative of a healthy environment, an unhealthy environment, or of questionable environmental health based on the shapes of the k-dominance curves for the reference samples.
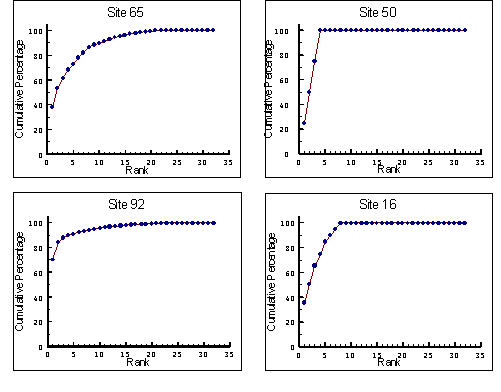
|
|
Figure 7.2 Plots of k-dominance curves for grab samples showing four different community patterns, Site 65 a typical curve, Site 50 a sample with low numbers of taxa, Site 92 a sample with low evenness, and Site 16 a sample without rare species.
|
7.2.1 Methods
The following community characteristics were incorporated into the analysis: Log(1+number of individuals), number of taxa and Menhinick Species Diversity Index (the number of species in the sample divided by the square root of the number of individuals). Features of the k-dominance curve selected for use in the analysis were the cumulative percentages of the first, third, fifth, and tenth most abundant species (species ranked by decreasing abundance) and cumulative percentages of the first, third, and fifth least abundant species (species ranked by increasing abundance).
7.2.2 Results
The k-dominance curves for all grab and dredge samples are presented in Appendix 3. A wide variety of curves exist. Four examples are presented in Figure 7.2. Site 65 is typical of a healthy community. The curve reaches 100% at species ranked 21 i.e. there were 21 taxa in the sample. The curve starts at 38% showing 38% of the total individuals belonged to species ranked first, the most abundant taxa present in the sample. This is high for many communities, but appears normal for south-eastern Australian estuarine communities. Examples for healthy marine and fiord communities in Clarke & Warwick (1994) were mostly between 10 and 20%. The curve gradually approaches 100% indicating there were a number of species with only one or two individuals, i.e. taxa that were rare at the site.
The curve for Site 50 reaches 100% at species rank 4, indicating only four taxa were in the samples. Because the cumulative percentage increases by 25% for each ranked species, we know there were the same number of individuals for each species, presumably one. This curve is indicative of a sample with low species richness or low density, which would suggest a stressed environment at the site where the sample was collected.
The curve for Site 92 starts at 70% indicating that most individuals in the sample belonged to the same taxon i.e. the sample had poor evenness. This is another indication of a stressed environment at the site where the sample was collected.
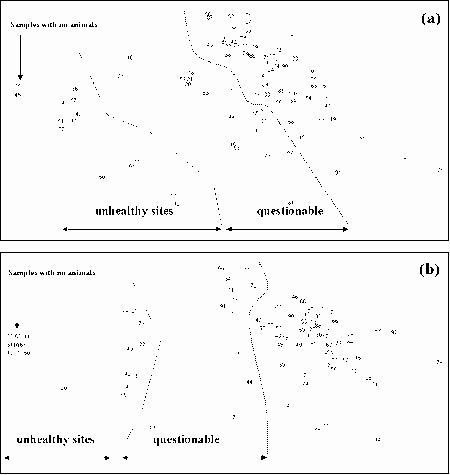
|
|
Figure 7.3 MDS plots for community and measurements from the k-dominance curves of a) grab samples, stress = 0.05 and b) dredge samples, stress = 0.04. Those samples whose k-dominance revealed they have poor evenness and/or low species richness have been marked as unhealthy sites. Another set of samples which have intermediate evenness and/or species richness have been marked as being of questionable health.
|
|
(original size image)
|
The curve for Site 16 rapidly climbs to 100% and turns sharply, not showing the gradual approach to 100% seen in the curve for Site 65. This suggests that there were no rare taxa in this sample. In actual fact the curve is caused by there being few individuals in the sample so that species ranked 4, 5, 6, 7 and 8 (each represented by one individual) added 5% on to the cumulative percentage. Sharp angles on reaching 100% exist for samples with less than 40 individuals. Thus they indicate the sites had low densities. Such curves are indicative of questionable environmental health at the site where they were collected.
The k-dominance curves can be used to identify samples that have low evenness and good species richness. Such a community would have been collected from a healthy environment. Also k-dominance curves reveal if the community had poor evenness and/or low species richness. This indicates the community was stressed and thus probably collected from a site with poor environmental health. The k-dominance curves for some sites, usually with low numbers of individuals, fell between these two groups. Thus conclusions about the environmental health of the site they were collected from could not be drawn. Sites with such samples are considered to be of questionable health.
The variables used in the ordination of the plots were selected to sort the samples mostly on their k-dominance curve shapes and to maximise the separation of samples with few individuals or few taxa from samples that had a large number of individuals and taxa. The resultant plot separates the samples along a gradient from azoic to those with complex community (Fig. 7.3). Because the ordination places samples with similar k-dominance curves close together, it provides an easy way to compare k-dominance curves.
Ordination of the k-dominance curve data illustrates the wide range of community structures encountered. Most of the samples are clustered together, indicating they had a typical estuarine community structure. A large proportion of the samples have been classified as representative of communities from unhealthy sites or sites where the environmental health is suspect (Fig. 7.3). Several samples were found to have more complex communities than the majority of sites. For the grab samples these were from Sites 76, 80, 91, 28 and 13. It could be expected that these would be samples with more of a marine nature than estuarine. However, this is not the case. Only Site 13 was in the lower estuary. Sites 76 and 80 were in the upper reaches of marine estuaries, i.e. they had high salinity but were not near the ocean. Sites 91 and 28 were in the mid-reaches of estuaries.
7.2.3 Discussion
This approach to assessing environmental health resulted in high proportions of reference samples being classified as unhealthy or of questionable health. This should not be seen as a failure of the method but rather an indication of the diverse and frequently stressed conditions encountered in our survey. Many of the samples we collected were azoic or depauperate. Any assessment of environmental health based on community characteristics will place these into an unhealthy category.
7.2.4 Testing Improving Estuarine Health, Tasmania
Macrofauna of the upper Derwent Estuary in Tasmania is very similar to that of south-eastern mainland Australia. In the upper reaches of the Derwent Estuary there is an outfall for the Boyer pulp and paper mill. Between 1990 and 1995 the mill's wastewater treatment plant was upgraded so as to minimise environmental impact on the receiving waterway.
In 1990, a survey of the subtidal macrobenthos found no benthic fauna in sediments between the outfall and 5 km downstream (Horwitz & Blake 1992). In 1995 and 1998 subsequent surveys were conducted to monitor how the abiotic and biotic environments had changed in response to the improved quality of the discharge (Moverley & Garland 1995; Garland & Moverley 1998).
In 1995, there had been substantial improvement to the benthic environment and only sites less than 400 m downstream of the mill showed a detectable impact (Moverley & Garland, 1995). However, these samples appeared to have been taken during a "good" year for benthos. Macrofauna densities were exceptionally high suggesting a time of low environmental stress and high productivity. During times when natural stress was higher a different pattern might exist. Such a change was observed in England as the Tees Estuary recovered after reduced pollution loading (Shillabeer & Tapp 1989). In 1998, densities were lower, but still similar to the highest densities recorded in the survey of mainland south-eastern Australian estuaries. This suggests conditions in 1998 were normal (Garland & Moverley 1998).
Multivariate analysis of k-dominance curves for these three surveys of the upper Derwent Estuary have been compared with the data from the mainland south-eastern Australian estuaries. As well as comparing the structure of the Derwent's macrobenthic community to that of mainland south-eastern Australia, this analysis also tests if the improvement to the health of the upper Derwent is detected by multivariate analysis of the k-dominance curves.
7.2.4.1 Results
For the 1990 samples (Figure 7.4a), 65% of k-dominance curves were in the portion of the MDS plot where their shapes suggested samples had been collected from an unhealthy environment. Twenty-seven percent were in the portion where it is uncertain from the shape of the k-dominance curves if the environment was healthy. Only 8% were in the portion of the MDS indicating a healthy environment. Compared to mainland south-eastern Australian estuaries, the upper and mid-reaches of the Derwent Estuary have had poor environmental health in 1990.
For the 1995 samples, ordination of the k-dominance curves (Figure 7.4b) suggests the environmental health of the upper and mid-Derwent Estuary was similar to that of south-eastern Australian estuaries (Table 7.1). For the 1998 samples, ordination of k-dominance curves (Figure 7.4c) suggests the environmental health of the upper and mid-estuarine sites in the Derwent was above that of the sites sampled in south-eastern Australian estuaries (Table 7.1).
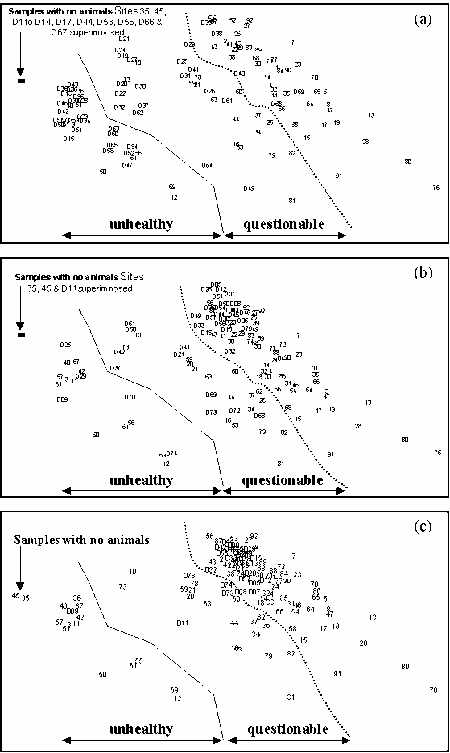
|
|
Figure 7.4 MDS plots for community and measurements from the k-dominance curves for grab samples from south-eastern Australian estuaries and the mid- and upper Derwent Estuary for a) 1990 b) 1995 and c) 1998. Derwent Estuary samples are prefixed with a D.
|
|
(original size image)
|
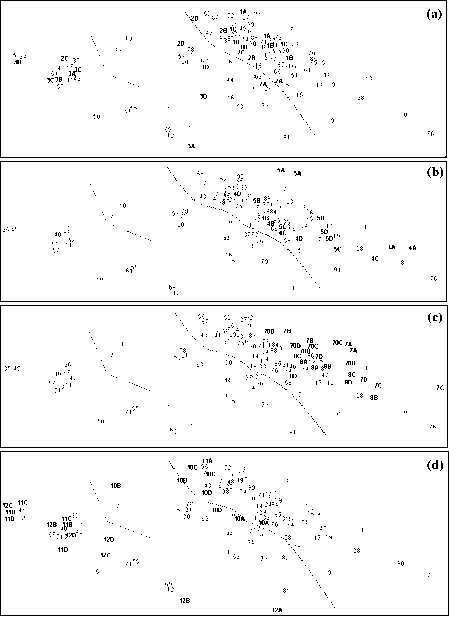
|
|
Figure 7.5 MDS plots for community and measurements from the k-dominance curves for grab samples from the main study and the temporal studies a) Hopkins b) Clyde c) Wingan and d) Darby. The first five and second five of the replicate samples were combined to give two samples of equivalent size to those used in the main study. These samples are labelled with the site number and either A, B, C or D indicating the time they were collected.
|
|
(original size image)
|
Table 7.1 Percentage of samples collected from sections of the MDS plot where shape of the k-dominance curve suggests the environment was unhealthy, questionable and healthy. Samples are SE Australia which were those collected for this study, Raw data for the Derwent Estuary for 1990 is from Horwitz & Blake 1992; 1995 is from Moverley & Garland 1995 and 1998 is from Garland & Moverley 1998). |
Sample | Unhealthy | Questionable | Healthy |
SE Australia | 17 | 21 | 64 |
Derwent 1990 | 65 | 27 | 8 |
Derwent 1995 | 15 | 25 | 60 |
Derwent 1998 | 4 | 20 | 76 |
Between 1995 and 1998, as well as changes in the number of samples that fit into the different regions of the MDS plot, there was also a shift of the area occupied by the majority of the curves. In 1995, mainland Site 56 was near the centre of the area occupied by most of the Derwent samples. This indicates most curves for this set of samples were similar to that of Site 56, a site at the mouth of the Fitzroy Estuary in western Victoria. For the 1998 samples, the k-dominance curves were lower and more to the right. Site 56 was on the outside of the area occupied by most of the Derwent samples. This indicates that not only had more sites developed a community indicative of a healthy environment but that in 1998 most of the samples had come from more complex species assemblages than 1995.
7.2.4.2 Discussion
The Derwent Estuary is not pristine. During the period of sampling, untreated primary and secondary treated sewage was being discharged into the lower and mid-reaches. There was heavy metal contamination of the sediments of the lower and mid-reaches, which was being regularly resuspended. The catchment area had been highly modified for agricultural use and the river flow is highly regulated by a series of dams for generating hydro-electricity. However, prior to the improvements to the Boyer pulp and paper mills wastewater treatment plant, the greatest perturbation for the upper estuary appears to have been the wood-fibres and water of high biological oxygen demand that was being discharged to the environment. Analysis of the k-dominance curves reveals that following the improved treatment for wastewater from the Boyer mill, there was a substantial improvement of environmental health in the mid- and upper Derwent Estuary.
Comparison of k-dominance curves revealed that in 1998 there was a lower proportion of sites in the mid- and upper Derwent Estuary from unhealthy sites or sites with questionable health than in the south-east Australian survey. This probably results from different estuarine classes possessing different patterns. In our study many of the sites with poor environmental health were in layered estuaries. The Derwent is a classical estuary. Classical estuaries may have more complex communities than some of the other estuarine classes. Therefore, this method for assessing estuarine health would probably be more accurate if the reference sites were drawn from the same class of estuary as the test sites.
7.2.5 Testing Temporal Patterns – South-eastern Australia
K-dominance curves for the nematode community have been used for assessing health of the Hunter Estuary at Newcastle, New South Wales (Hodda & Nicholas 1986). They found that the curves were not temporally stable with transient changes caused by seasonal patterns in the density of the commonest species and opportunistic species. Many nematode species have life cycles much shorter than macrofauna, consequently this problem may not exist for k-dominance curves of estuarine macrofauna. The temporal samples have been used to examine variability in k-dominance curves for south-eastern Australian estuarine macrofauna.
7.2.5.1 Methods
So that the samples would be comparable to those used in the major study, the replicate samples have been combined in sets of five. Where there were 10 replicates two samples were formed by combining the first and second five replicates. Where there were eight or nine replicates the first five were combined to give a sample and one or two from the first five were added to the remainder to form the second sample. If there were less than eight replicates only the first five were combined to give one sample.
7.2.5.2 Results
Different temporal patterns were observed for different estuaries and sometimes for the sites within an estuary (Fig. 7.5). However, it was rare for the changes to result in sites moving from one class to another (e.g. healthy to questionable). Two cases, Sites 11 and 12, were both in the Darby, the freshwater estuary. Reasonable numbers of individuals were only collected from these sites in the first survey. Subsequent samples were depauperate. Consequently, for surveys two, three and four the k-dominance curves indicate the environment was unhealthy. The ordination of k-dominance curves for Sites 2 and 10 fell on the boundary between curves indicating questionable health and healthy sites. Although these two sites have a number of samples in different classes, it is not so much a function of temporal variability in the k-dominance curves but a result of the region of the ordination the curves were located in.
Sites 8 and 70, both in Wingan Inlet (Fig. 7.5c), had similar distances between replicates and samples collected at different times, indicating there was no detectable temporal difference in the k-dominance curves. Sites 1 (Fig. 7.5a) and 7 (Fig. 7.5c) showed relatively minor temporal changes. Sites 4 and 5, both in the Clyde (Fig. 7.5b) showed a substantial difference between the k-dominance curves collected in the first survey and the later surveys, but all curves indicated a healthy environment. Sites 11 and 12, both in the Darby (Fig. 7.5d) also had a substantial difference between the curves for the first survey and subsequent surveys. These were two sites where the differences resulted in a change in health status for the sites. The curve for the first set of samples suggested Site 11 was healthy and Site 12 was of questionable health. Subsequent samples suggested these sites were unhealthy.
Most of the Site 2 (Fig. 7.5a) replicates fell on the boundary between sites with questionable health and healthy sites. However, there was a large vertical spread indicating major temporal changes in the k-dominance curve shapes. The two replicates from the third survey (2C) are widely dispersed, one indicating an unhealthy environment and the other a healthy environment. This resulted from random placement of the grab during the collections. When the third set of samples was collected, most of the sediment at Site 2 was anoxic. However, there were small mounds of calcareous polychaete tubes rising above the anoxic silt. These were high enough above the silt to allow animals to survive in the anoxic conditions. Two or three of these mounds were sampled in the second set of five replicates. One was particularly well populated with 41 individuals in 8 taxa, and the others had 6 individuals in 4 taxa and 17 individuals in 2 taxa. The most individuals that were found in a sample belonging in the first five replicates was four. Consequently, due to random variation, when assessing environmental health, these two replicates gave very different results. Site 10 (Fig. 7.5d) also had two replicates (10B) that were widely separated. Similarly the reason for this was random variation with one replicate having six taxa. The most in any other replicate was 3.
7.2.5.3 Discussion
The analysis reveals that for most sites, k-dominance curves were temporally unstable. However the changes were such that they had little impact on the features of the curves that had been used to assess the health of the location where the samples had come from. Therefore, temporal instability does not appear to be an impediment for using this method to assess environmental health.
The results also highlight that in 5% of cases (2 of 41) there was a substantial difference in the assessment from replicate samples, and that these were due to random variability in replicate grabs when multiple grabs were being collected. It may be possible to reduce this error rate by increasing the sample size. However, to some extent it will always be a problem and for any monitoring program some method of assessing error rate should be included.
7.2.6 Testing Temporal Patterns – Central Queensland
Between November 1974 to May 1983 benthos in the Calliope Estuary at Gladstone in Central Queensland was sampled at approximately three monthly intervals. Data for these samples were published in Moverley & Saenger (1986). This provides a long-term data set to test for the temporal stability of k-dominance in a subtropical environment and for comparing the curves from a subtropical estuary against those from south-eastern Australian.
Prior to commencing the Gladstone survey the river had been subjected to severe flooding as three cyclones moved down the coast in six weeks and on a single day discharge of the Calliope River exceeded the annual average. For the first four or five years of the study the benthos was progressively increasing in density and species richness presumably as part of the recovery from this flooding as recolonisation and restructuring of the sediment occurred (Moverley et al. 1986).
Table 7.2 Months when benthic surveys of the Calliope were conducted (Moverley & Saenger, 1986). |
Survey | Month | Survey | Month |
1 | Nov 74 | 17 | Apr 79 |
2 | Mar 75 | 18 | Jly 79 |
3 | Jun 75 | 19 | Nov 79 |
4 | Oct 75 | 20 | Mar 80 |
5 | Feb 76 | 21 | Jun 80 |
6 | May 76 | 22 | Oct 80 |
7 | Aug 76 | 23 | Feb 81 |
8 | Nov 76 | 24 | Jun 81 |
9 | Feb 77 | 25 | Sep 81 |
10 | May 77 | 26 | Dec 81 |
11 | Oct 77 | 27 | Mar 82 |
12 | Jan 78 | 28 | Aug 82 |
13 | Mar 78 | 29 | Nov 82 |
14 | Jly 78 | 30 | Jan 83 |
15 | Nov 78 | 31 | May 83 |
16 | Jan 79 | | |
7.2.6.1 Methods
Samples for the Gladstone study were collected with a 0.05 m2 Van Veen grab. Two grab samples were combined to give a total area of 0.1 m2. Data was extracted from Moverley & Saenger (1986). Dates when samples were collected are given in Table 7.2. Data for two sites are presented, Transect 1 Site 2, which was in the channel near the mouth of the estuary, and Transect 6 Site 3 the furthest upstream transect sampled and a site within the channel. Sample 31 was collected just after a minor flood.
7.2.6.2 Results
For Transect 1 Site 2 the first five samples are in the section of the ordination indicating the environmental health of the site was questionable and most of the remaining samples are in the section of the plot indicating a good environmental health (Fig. 7.6a). This suggests the site is not usually stressed but at the start of the sampling program was stressed by the prior severe flooding.
Three samples did not fit this pattern. Samples 10 (May 1977) and 18 (July 1979) were in the section of the ordination with questionable health, and Sample 31 (May 1983) in the section of the ordination indicative of poor environmental health. Not all samples collected in late autumn or winter indicated a state of poor environmental health, indicating this was not a seasonal pattern.
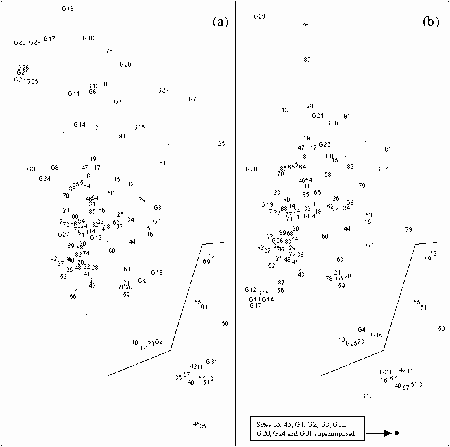
|
|
Figure 7.6 MDS plots for community and measurements from the k-dominance curves for samples collected in south-eastern Australia and samples collected between 1974 and 1983 from the Calliope Estuary, Gladstone, Central Queensland (Moverley & Saenger1986) a) Transect 1 Site 2, near the mouth b) Transect 6 Site 3, an upper estuarine site. Gladstone samples are labelled with a G and a number 1 to 31 giving the survey they were collected in.
|
|
(original size image)
|
Transect 6 Site 3 also appeared to have been impacted by the severe flooding of 1974, with the first six samples (i.e. up to May 1976) indicating the site was unhealthy or of questionable health. The remaining samples were scattered around the ordination plot in a similar pattern to that for the south-eastern Australian estuaries. Twenty-four percent were in the portion of the ordination indicating poor health, 20% in the portion indicative of questionable health and the rest spread throughout the area indicative of healthy environmental conditions. From Table 7.3 it can be seen that all but one of the months when the k-dominance curves for Transect 6 Site 3 indicated poor health were in the first six months of the year. This suggests poor environmental health of the Calliope estuary may be climate related (Gladstone receives most of its rainfall in summer).
Table 7.3 Months when samples from Transect 6 Site 3 indicated poor or good environmental health excluding the first six surveys, because these were affected by severe flooding in 1974. |
Poor Health | Good Health |
Survey | Month | Survey | Month |
9 | Feb 77 | 7 | Aug 76 |
10 | May 77 | 8 | Nov 76 |
13 | Mar 78 | 11 | Oct 77 |
16 | Jan 79 | 12 | Jan 78 |
18 | Jly 79 | 14 | Jly 78 |
20 | Mar 80 | 15 | Nov 78 |
23 | Feb 81 | 17 | Apr 79 |
24 | Jun 81 | 19 | Nov 79 |
25 | Sep 81 | 21 | Jun 80 |
27 | Mar 82 | 22 | Oct 80 |
31 | May 83 | 26 | Dec 81 |
| | | 28 | Aug 82 |
| | | 29 | Nov 82 |
| | | 30 | Jan 83 |
7.2.6.3 Discussion
The results indicate k-dominance curves for a subtropical estuary are similar to those for the south-eastern Australian estuaries. Although it would be best if comparisons are made on a regional basis, these results do indicate that a database of k-dominance curves could be used to compare community structure over broad geographical regions, and provide a ready assessment of environmental health.
At the time of sampling the Calliope Estuary was not pristine. Gladstone's sewage was being discharged in the estuary and cooling water from a power station was being discharged into the mid-estuarine reaches. The two sites presented here were as far upstream and downstream from these sites as was sampled. And at sites closer to the outlet impacts could not be detected. Therefore the changes observed here are probably natural. There was temporal variability in the data and the Calliope Estuary appears to be much more prone to poor health in the first six months of the year. Poor health was more frequently recorded in the upper estuary than near the mouth.
7.2.7 Assessment of k-dominance curves
The results presented here indicate multivariate analysis can be used to group samples with similar k-dominance curves and that the ordination plot can be divided into areas where the curves indicate the samples came from sites which were unhealthy or of questionable health. Because the k-dominance curves are independent of species curves they can be used to compare samples from outside the area where the reference samples were collected. K-dominance curves are also not method dependent so they can be used to compare samples that have been collected by different methods.
The multivariate analysis of k-dominance curves was found to be a useful method to assess the environmental health of test sites and to compare them with the average health for sites in south-eastern Australian estuaries. However, the results suggest different classes of estuaries and perhaps different regions have different proportions of sites with poor or questionable health. Therefore, before this method could be developed for use on a national basis, databases of reference samples from different classes of estuaries and different regions need to be compiled.
Chapter 8: Conclusions and recommendation
- 8.1 RIVPACS-type approach
- 8.2 ABC evaluation
- 8.3 Estuarine Health Assessment Protocol
There were four objectives for this project.
- To evaluate the utility of a RIVPACS-type approach to estuarine health assessment and to investigate the appropriate taxonomic resolution to be used in a standard protocol.
- To collect new data from estuaries to support a demonstration RIVPACS-type model.
- To evaluate the ABC (Abundance Biomass Comparison) method of health assessment if suitable existing data are available
- To develop a nationally applicable standard protocol for estuarine health assessment using benthic macrofauna.
8.1 RIVPACS-type approach
The evaluation of the RIVPACS-type approach to estuarine health assessment required two questions to be answered. Can a RIVPACS-type model be developed for Australian estuaries and are these of use for assessing environmental health?
Our work demonstrated a RIVPACS-type model could be developed for south-eastern Australian estuarine macrobenthic communities and thus presumably for all Australian estuaries. Only 58 reference sites were used in developing this model, which had a 70% accuracy for predicting expected taxa at test sites. The two-way table analysis of the grab and dredge data sets revealed there were many single sites or pairs of sites with unique sets of taxa. Because such taxa were usually collected at fewer than three sites they were excluded from construction of our model. These samples were frequently collected from sites in the lower reaches of open estuaries or from low salinity areas in the upper estuarine reaches. These types of habitats were poorly represented in our survey. By targeting such habitats, sampling for a large-scale model could include more sites representative of these communities. Presumably by such actions and with hundreds of reference sites a large-scale model would be more accurate.
Four major problems were encountered in applying a RIVPACS-type model to estuarine health assessment. These are choice of reference sites, the large range of macrofauna density found at different sites, low numbers of taxa predicted for test sites, and unpredictable temporal changes in estuarine benthic communities.
Most Australian estuaries have been subjected to some degree of anthropogenic impact. Consequently, reference sites can not be located at pristine or near pristine locations so a RIVPACS-type model can only be based on reference sites in their present state. This could lead to difficulties in interpreting RIVPACS-type model outputs, particularly when the number of observed taxa at a test site exceeds the predicted number. Such results could indicate some environmental disturbance or that the test site is of higher environmental quality than the reference sites.
A large proportion of the sites we sampled had few individuals and there was a high probability that common taxa were missed at some sites. This occurred because there was a considerable reduction in macrobenthos density between the pilot survey and the main survey and the wide range of densities encountered. Methods to increase the numbers of individuals sampled from low-density sites are discussed below.
Our model which was based on combining grab and dredge samples collected at one time predicted between seven and 18 taxa per test site. This is a low number of predicted taxa for using in a RIVPACS-type model for assessing environmental health. With such low numbers of expected taxa a model would not be very sensitive to environmental change. A relatively large proportion of the community would have to be lost before the changes could be detected. Sampling fauna from a wider size range, collecting larger samples and changing the time of sampling may address this problem.
Our model was based on macrofauna, that did not pass through a 1 mm mesh sieve. Our experience is that collecting more macrobenthos by using a smaller mesh, say 0.5 mm, has little impact on the numbers of taxa. The extra animals collected are usually juveniles of species already identified. Also, if identifications are to species level, the additional individuals often can not be reliably identified because they are not mature. In estuarine samples there are usually large amounts of detritus in the sediments so reducing the sieve size increases sorting time. For estuarine samples it would be more productive to collect larger samples on a 1 mm mesh than to use a smaller mesh sieve.
Combining meiofauna (specimens collected on a 62 m
m mesh sieze) and macrofauna samples would undoubtedly increase the numbers of taxa. Moverley (1995) demonstrated it is possible to undertake Australian ecotoxicological studies using meiofauna with the keys presently available. However, investigating meiofauna was not within the scope of the present study. We recommend that the potential for using meiofauna to assess estuarine health be investigated.
There are a number of ways sample size could be increased. Techniques used in monitoring river health usually combine three sets of samples collected from different times of the year. This trebles the sample size and adds taxa that may not be present at a site all year round. An impediment to this for estuarine samples is that the variables used in the model need to be temporally stable. Our model included salinity, which at any estuarine site would be subject to short and long-term oscillations. Salinity could probably be replaced by a variable that was related to a site's position within an estuary such as percentage of estuarine length from mouth. However, this would require defining the upper and lower boundaries of the estuaries by values, which are temporally stable.
Increasing the sample size is not recommended as a method for increasing the numbers of taxa in the samples. This is because of the wide range of densities of macrobenthos encountered in estuaries. Numbers of animals in our samples ranged over four orders of magnitude. If the sample size were increased much of the additional effort would go into sorting and identifying specimens from samples where there were high numbers of individuals, and the community already well defined. Instead we recommend a fixed-count method be investigated in which larger samples are collected and subsampled and a set number of individuals from each site identified. This would result in greater effort being spent on recording taxa present at sites with low densities.
It may be possible to increase the numbers of individuals and taxa in samples by changing the time of year when samples are collected. Samples for our model were collected in March and April. When our pilot study was done in December 1976, densities were almost three times that of the main survey. Species accumulation curves indicated that there would have been twice as many taxa in the samples if the main survey had been done in December. Seasonal and other temporal patterns in Australian estuaries are poorly documented. If the pattern we observed was true for every year, a model based on December samples would have more predicted taxa for test sites than the model based on March/April data.
The impact of temporal changes on estuaries is potentially the greatest problem with using a RIVPACS-type approach to assessing estuarine health. The temporal differences observed in species accumulation curves indicate there would be temporal changes in the probability of collecting different taxa. If the changes were seasonal a RIVPACS approach could be used provided that reference and test samples were collected at the same time of the year. However, Jones' (1987) and Poore's (1982), studies of large estuaries found that temporal changes in abundance of individuals and species richness in south-eastern Australian estuaries are not seasonal. If true for all estuaries, reference site data would need to be collected every time it was desired to test a site. Such an approach would make use of a RIVPACS-type models impractical.
Using a fixed-count approach to sorting the samples may solve this problem. Instead of predicting the probability of collecting a taxon in a sample the probability would be for identifying the taxa in the set number of individuals. If the community structure remains the same and species accumulation is only changing temporally because densities are changing, a RIVPACS approach could be used because numbers of taxa for a set number of individuals should be similar for samples collected at different times.
We recommend further studies be undertaken to document the relative significance of seasonal and year to year changes in small south-eastern Australian estuaries. Also, we recommend studies be undertaken to see if adopting a fixed-count approach to sorting samples for an estuarine health model can be used to eliminate changing probabilities for collecting taxa at a site with temporal changes to macrobenthos density.
The present data set found that there was little difference in using either species or family level taxonomic identifications. However, if sample size is changed and more individuals sorted from sites with low densities this may change. Therefore we recommend that future work be based on species level identifications and this be reassessed after the problems with low numbers of individuals and expected taxa in the samples have been addressed.
There is potential for using a RIVPACS-type approach for monitoring estuarine health. However, before the protocols for a national sampling program can be drawn up there is a need for greater research into temporal changes in benthic communities and the impacts of using fixed count sampling and addition of multiple sets of samples collected at different times of the year for developing the reference database must be investigated.
8.2 ABC evaluation
No suitable data sets were available for testing the ABC method for evaluating estuarine health. The ABC method does not reveal if a sample has been anthropogenically stressed but if it has been disturbed either naturally or by man. Considering that estuaries are naturally stressed environments it would be expected that all samples would be positive. Therefore, we did not believe collecting samples to test the ABC method in Australian estuaries was warranted.
K-dominance curves use the abundance curves of the ABC method. We showed that multivariate analysis of a large number of k-dominance curves could be used to identify sites where the macrobenthic community indicated the environment was healthy, unhealthy or of questionable health. It was demonstrated that the method was applicable for samples collected over a wider range than just mainland south-eastern Australia and that they were independent of the sampling method.
Environmental quality of estuarine sites varied spatially and temporally and as a consequence some communities appear to suggest poor environmental health while not suffering from anthropogenic stress. This is probably characteristic of many Australian estuaries from time to time and will give false indications for any bioassessment method.
Multivariate analysis of k-dominance curves can be used to identify sites for detailed study to see if they are anthropogenically stressed. For regional and single estuaries they can be used to see if the proportions of sites with communities indicative of poor or questionable health are changing. We therefore believe the multivariate analysis of k-dominance curves is an appropriate method for assessing the environmental health of estuarine sites, and recommend a database of reference sites for comparison of different estuary classes in different geographic locations be established.
8.3 Estuarine Health Assessment Protocol
A number of difficulties, which were beyond the scope of this project, need to be resolved before a national estuarine health assessment protocol can be established.
The term "estuary" needs to be defined for this purpose. In Australia as well as classical estuaries there are coastal lagoons, brackish coastal lakes, seasonally closed estuaries and other estuaries whose opening is less predictable. Salinities may be brackish or hypersaline. Many people believe that estuaries only exist where there are tidal fluctuations and mixing of sea-water and fresh water. In their opinion closed and hypersaline estuaries are not estuaries. At the time of our study many of the open estuaries had salinity the concentration of sea water. Should such bodies of water be included in a national estuaries biomonitoring program or should biomonitoring only be limited to classical estuaries?
Bucher & Saenger (1989) provided a definition of an estuary, as a semi-enclosed bay where the distance between banks, including intertidal mudflats was less than 2 km apart. However, included in their catalogue are bodies of water like Port Phillip Bay, where the banks are separated by more than 4 km. We also believe this definition is inappropriate for estuarine health management because it combines rivers with separate catchments that flow into a complex estuary into the same estuary. We believe it would aid future management if estuarine health assessment was conducted separately for the different streams of a complex estuary. This would be aided if the separate streams of a complex estuary were recognised as different estuaries.
Twenty-five percent of the sites we sampled in randomly selected estuaries possessed a halocline. In these cases there was long-term stratification with a pool of low salinity water floating on a pool of high salinity water with very little mixing. This is different to what is normally referred to as a layered estuary where there is a vertical salinity gradient with salty water moving up the bottom layers and brackish water flowing out in the upper layers. In these estuaries the waters are constantly being replenished and mixing of the upper and lower layers is occurring. Many sites in estuaries we classified as layered had depauperate macrobenthic communities indicating a stressed environment. In the analysis of k-dominance data it was shown that sites in Central Queensland's Calliope Estuary also sporadically have communities indicative of poor environmental health. Presumably this arises from natural stresses on the estuarine community such as floods and deoxygenation. As bioassessment looks for ecological signals of anthropogenically-stressed environments the protocol must interpret whether the data indicate natural or anthropogenic influence.
Before a national protocol can be developed optimal sample strategy needs to be determined. As most of the expense is for the sorting and identification of animals, sample size has a major impact on costs for undertaking such work. Samples need to be large enough to adequately describe the community but not so large that considerable time is wasted in sorting unnecessary individuals. Ideally, samples should have at least 100 individuals and sorting of more than 300 is probably excessive.
As is normally the case with marine bioassessment, we chose to sort samples from equal surface areas and identify all specimens. Two problems related to sample size arose. First many of our samples had too few individuals to adequately describe the community. We had undertaken a pilot survey to ascertain sample size but during the three months of sorting these samples and preparing for the major survey, macrofauna density decreased by two-thirds. Consequently, major survey samples were smaller than desirable.
The second problem was due to the large range of densities encountered. Numbers of individuals in our samples ranged over four orders of magnitude. Thus for some samples time was wasted by sorting samples that had up to 3,000 individuals.
An alternative to the equal area sample method is the fixed count method in which subsamples are sorted until a set number of individuals have been collected. This approach has been adopted by meiofaunologists who regularly encounter samples with densities of fauna spread over a few orders of magnitude. The United States Environmental Protection Agency uses this method for their Rapid Bioassessment technique of rivers and the AusRivAS study uses fixed counts. A fixed count would reduce the effort put into sorting and identifying animals at sites with dense meiofauna, and put greater effort into finding more individuals at low density sites. Consequently, it would probably be more appropriate than the equal sample size method for assessing estuarine health.
Forty percent of the taxa in our samples were only collected at one site and 75% at five or fewer sites. Two-way table analysis revealed there were a large number of sites with unique species groups. When using multivariate analysis, including RIVPACS-type models, this leads to problems because rare species contribute little to community analysis but add noise to statistical solutions (Gauch 1982). In constructing our model we excluded taxa that did not occur in at least three samples. Because more taxa would be recorded from sites where densities were low, fixed counts should increase the numbers of sites different taxa were recorded from. However, it must be realised that low species richness and presence of many rare taxa is a basic characteristic of estuarine communities and therefore samples will always reflect this. Consequently, a RIVPACS-type model of estuaries will always have lower numbers of expected taxa than the models for rivers.
Our work indicated that dividing the estuaries into classes on characteristics relative to the physicochemical environment of the macrobenthos would allow better assessment of estuarine health. Macrobenthic communities in estuaries we classified as layered were frequently depauperate and thus make interpretation of what is a healthy community difficult. We strongly recommend that such estuaries be excluded from environmental health assessment compared to estuaries with well mixed water columns.
Developing a classification system of estuaries was not in the scope of our project. We describe five estuarine classes relevant to south-eastern Australia observed during our study. We were not able to investigate how temporal changes in river flows affected these classes and if a greater range of classes are required to cover the whole of Australia. We believe it is necessary to have a national estuary classification system based on characteristics relevant to the macrobenthos before drawing up a National Estuarine sample protocol. This would allow different methods to be used where appropriate or to exclude certain estuarine classes where the macrobenthic community may be under severe natural stress.
Our work also revealed there were temporal differences in the macrobenthic communities which extended over the whole of south-eastern Australia. If these changes can not be predicted, interpreting whether small changes to estuarine macrobenthic community are due to deteriorating or improving environmental health is impossible. We therefore feel that before national protocols to assess estuarine health can be drawn up more work needs to be undertaken to develop some understanding of these temporal changes to investigate if sampling times can be optimised to minimise temporal impacts.
Because of the large number of problems that need to be solved before a standard protocol for estuarine health assessment can be developed, we have not undertaken the development of such a protocol.
9. References
- Belbin, L., 1993. PATN pattern analysis program. CSIRO Division of Wildlife and Ecology, Australia
- Bird, E.C.F., 1967. Depositional features in estuaries and lagoons on the south coast of New South Wales. Australian Geographical Studies, 5:113-124.
- Bowden, M.W., 1996. An overview of the national estuary program. Natural Resources and Environment, 11:35-38.
- Briggs, I., N. Carrick, H. Fisher, D. Milledge & E. Williams, 1980. A survey of the estuarine fauna of Eurobodalla Shire. Australian Museum. Sydney.
- Bucher, D.J. & P. Saenger, 1989. An inventory of Australian estuaries and enclosed marine waters. Report and database prepared for the Australian Recreational Fishing Confederation of Australia, National Parks and Wildlife Service and the Centre for Coastal Management Lismore, NSW.
- Cameron, W.M. & D.W. Pritchard, 1963. Estuaries. pp 306-324 In The Sea, edited by M.N. Hill; Vol. 2. John Wiley and Sons, New York.
- Cao, Y., A.W. Bark & W.P. Williams, 1996. Measuring the responses of macroinvertebrate communities to water pollution: a comparison of multivariate approaches, biotic and diversity indices. Hydrobiologia, 341:1-19.
- Cao, Y., D.D. Williams & N.E. Williams, 1998. How important are rare species in aquatic community ecology and bioassessment. Limnology and Oceanography, 43:1403-1409.
- Castel, J., P.-J. Labourg, V. Escaravage, I. Auby & M.E. Garcia, 1989. Influence of seagrass beds and oyster parks on the abundance and biomass patterns of meio- and macrobenthos in tidal flats. Estuarine and Coastal Marine Science, 28:71-86.
- Clarke, K.R. & R.M. Warwick, 1994. Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, U.K.
- Costanza, R., W.M. Kemp & W.R. Boynton, 1996. Predictability, scale, and biodiversity in coastal and estuarine ecosystems: Implications for management. Ambio, 22:88-96.
- Davies, P.E. (ed.) (1994) Monitoring river health initiative river bioassessment manual. National river processes and management program, Freshwater Systems, Tasmania.
- Day, J.H., 1981. Estuarine ecology, with particular reference to southern Africa. Balkema, Rotterdam.
- Day, J.W. Jr., C.A.S. Hall, W.M. Kemp & A. Ya-ez-Arancibia, 1989. Estuarine Ecology. John Wiley, New York.
- Deeley, D.M. & E.I. Paling, 1999. Assessing the ecological health of estuaries in Australia National River Health Program, Urban Sub Program, Report No 10, LWRRDC Occasional Paper 17/99. Land and Water Resources Research and Development Corporation Canberra.
- Digby, M.J., P. Saenger, M.B. Whelan, D. McConchie, B. Eyre, N. Holmes & D. Bucher, 1988. A physical classification of Australian estuaries. Report prepared for the urban water research association of Australia by the Centre for Coastal Management, Southern Cross University, Lismore, New South Wales. pp 47.
- Edgar, G.J., N.S. Barrett & D.J. Graddon, 1998. A classification of Tasmanian estuaries and assessment of their conservation significance: an analysis using ecological and physical attributes, population and land use Report to Environment Australia From Parks and Wildlife Service, Dept of Environment & Land Management. Hobart. 1-188.
- Elliott, M. (1997), What is an estuary? – The interface between environmental science and the law. Estuarine and Coastal Sciences Association Bulletin, 24:3-4
- Eyre, B. (1998), Transport, retention and transformation of material in Australian estuaries. Estuaries, 21:540 - 551.
- Garland C.D. & J.H. Moverley, 1998. Continued recovery of macrobenthos in sediments of the upper Derwent River Estuary in relation to reduced woodfibre enrichment.. Report prepared for ANM Ltd, Boyer. Aquahealth, University of Tasmania, Hobart.
- Gauch, H.G., 1982. Multivariate analysis in community ecology Cambridge University Press 298 pp.
- Gauch, H.G. & R.H. Whittaker, 1981. Hierarchical classification of community data. Journal of Ecology, 69:537-557.
- Growns, J.E., B.C. Chessman & P.K. McEvoy, 1995. Rapid assessment of rivers using macroinvertebrates: Case studies in the Nepean River and Blue Mountains, NSW. Australian Journal of Ecology, 20:130-141.
- Harrison, P.G. & K.H. Mann, 1975. Detritus formation from eelgrass Zostera marina (L.): the relative effects of fragmentation, leaching and decay. Limnology and Oceanography, 20:924-934.
- Hodda, M. & W.L. Nicholas, 1986. Nematode diversity and industrial pollution in the Hunter River Estuary, NSW, Australia. Marine Pollution Bulletin, 17:251-255.
- Horwitz, P. and G. Blake (1992). The benthic macrofauna of sludge-affected sediments in the Derwent Estuary, Southern Tasmania. Proc. R. Soc. Tasman. 126:67-72.
- Johnson R. K. and T. Wiederholm (1989). Classification and ordination of profundal macroinvertebrate communities in nutrient poor, oligo-mesohumic lakes in relation to environmental data. Freshwater Biology 21: 375-386.
- Jones, A.R., 1987. Temporal patterns in the macrobenthic communities of the Hawkesbury Estuary, New South Wales. Australian Journal of Marine and Freshwater Research, 38:607-624.
- Jones, A.R., C.J. Watson & A. Murray, 1986. Spatial patterns in the macrobenthos of the Hawkesbury Estuary, New South Wales. Australian Journal of Marine and Freshwater Research, 37:521-543.
- Ketchum, B.H., 1983. Ecosystems of the World Vol. 26 Estuaries and Enclosed Seas. Ed. in Chief Goodall. Elsevier, New York.
- Kinne, O., 1967. Physiology of estuarine organisms with special reference to salinity and temperature: general aspects. In Estuaries, ed G.H. Lauff. Publication No. 83 American Association for the Advancement of Science, Washington. 525-540.
- Lauff, G.H., 1967. Introduction. In Estuaries, ed G.H. Lauff. Publication No. 83 American Association for the Advancement of Science, Washington. v-vi.
- Levinton, J.S. & S. Stewart, 1988. Effects of sediment organics, detrital input, and temperature on demography, production and body size of a deposit feeder. Marine Ecology Progress Series, 49:259-266.
- Marchant R., A. Hirst, R. H. Norris, R. Butcher. L. Metzelling and D. Tiller (1997). Classification and prediction of macroinvertebrate assemblages from running waters in Victoria, Australia. Journal North American Benthological Society 16: 664-681.
- McLusky D.S., 1993. Marine and estuarine gradients - an overview. Netherlands Journal of Aquatic Ecology, 27:489-493.
- Montagna, P.A. & R.D. Kalke, 1992. The effect of freshwater inflow on meiofaunal and macrofaunal populations in the Guadalupe and Nueces Estuaries, Texas. Estuaries, 15:307-326.
- Morrisey, D., 1994. Estuaries, in Coastal Marine Ecology of Temperate Australia. Eds. A.J. Underwood & M.G. Chapman. UNSW Press, Sydney. 152-170.
- Moss D., M. T. Furse, J. F. Wright, and P. D. Armitage (1987). The prediction of the macro-invertebrate fauna of unpolluted running-water sites in Great Britain using environmental data. Freshwater Biology 17: 41-52.
- Moverley, J.H., 1995. Temporal and spatial effects of organic enrichment on meiobenthic communities. PhD Thesis, University of Tasmania, Hobart.
- Moverley, J.H. & C.D. Garland, 1995; Recovery of macrobenthos in sediments of the upper Derwent River Estuary in relation to reduced wood fibre enrichment. pp. 18. Report prepared for ANM Ltd, Boyer. Aquahealth, University of Tasmania, Hobart.
- Moverley, J & A. Jordan, 1996; Biological surveys of inshore soft-bottom communities in Tasmania. OR2000 Project No. OR02. Report to Ocean Rescue 2000. Department of Primary Industries and Fisheries Tasmania, Hobart.
- Moverley, J.H. & P. Saenger, 1986. Benthic Collection data for the Calliope River and Auckland Creek Estuaries, Gladstone, November 1974 to May 1983 Queensland Electricity Commission, Technical Report 86/03. Brisbane.
- Moverley J.H., P. Saenger and M.A. Curtis, 1986; Patterns of polychaete recolonisation in a Queensland subtropical estuary following severe flooding. Hydrobiologia, 134 pp. 227-235.
- Norris, R.H. & A. Georges, 1993. Analysis and interpretation of benthic macro-invertebrate survey. In Freshwater biomonitoring and benthic macroinvertebrates, Eds D.M. Rosenberg & V.H. Resh. Chapman & Hall, New York. 234-286.
- Parsons M. and R. H. Norris (1996). The effect of habitat-specific sampling on biological assessment of water quality using a predictive model. Freshwater Biology 36: 419-434.
- Platt, H.M., K.M. Shaw & P.J.D. Lambshead, 1984. Nematode species abundance patterns and their use in the detection of environmental perturbations. Hydrobiologia, 118:59-66.
- Poore, G.C.B., 1982. Benthic Communities of the Gippsland Lakes, Victoria. Australian Journal of Marine and Freshwater Research, 33:901-915.
- Poore, G.B. & J.D. Kudenov, 1978. Benthos of the Port of Melbourne: the Yarra River and Hobsons Bay, Victoria. Australian Journal of Marine and Freshwater Research, 29:141-155.
- Ranasinghe, R. & C. Pattiaratchi, 1999. Circulation and mixing characteristics of a seasonally open tidal inlet: a field study. Marine and Freshwater Research, 50:281-290.
- Reynoldson T. B., R. C. Bailey, K. E. Day, and R. H. Norris (1995). Biological guidelines for freshwater sediment based on Benthic Assessment of SedimenT (the BEAST) using a multivariate approach for predicting biological state. Australian Journal of Ecology, 20: 198-219.
- Roy, P.S., 1984. New South Wales Estuaries: Their Origin and Evolution. In Coastal Geomorphology in Australia. B.G. Thom (ed). New York Academic Press, New York. 99-121.
- Shillabeer, N. and J.F. Tapp (1989). Improvements in the Benthic Fauna of the Tees Estuary after a Period of Reduced Pollution Loading. Marine Pollution Bulletin, 20:119-123.
- Simpson J., R. H. Norris, L. Barmuta and P. Blackman (1996). AusRivAS. National River Health Program. User Manual.
Appendix 1 Abiotic Effects on Univariate Indices
- A1.1 Geographical Location
- A1.2 Depth
- A1.3 Sediment Organic Content
A1.1: Geographical Location
A1.2 Depth
A1.3 Sediment Organic Content
Appendix 2: Two-Way Tables
- Two-way table of grab sample data
- Two-way table of dredge sample data
Table A2.1 Two-way table of grab sample data. Data has been independently standardised by the number of individuals in samples. The symbol . has been used to indicate absence of a species at a site. The symbols -+o* have been used to indicate four categories of increasing proportion of the species in a sample. Two sites with no fauna have been excluded from analysis.
Table A2.2 Two-way table of dredge sample data. Data has been independently standardised by the number of individuals in samples. The symbol . has been used to indicate absence of a species at a site. The symbols -+o* have been used to indicate four categories of increasing proportion of the species in a sample. Seven sites with no fauna have been excluded from analysis.
These tables have been saved using Sun Star Office. They may be downloaded either in the Sun Star Office Text Format or MS Word 97/2000 format.
 Appendix 2 Sun Star Office Writer Format (0.15 m-bytes)
Appendix 2 Sun Star Office Writer Format (0.15 m-bytes)
 Appendix 2 MS Word 97/2000 Format (0.11 m-bytes)
Appendix 2 MS Word 97/2000 Format (0.11 m-bytes)
Appendix 3: Model Predictions
- Wingan Inlet, lower (EH07)
- Merriman Creek, upper (EH18)
- Lake Tyers, mid (EH29)
- Lake Yambuk, upper (EH53)
- Curalo Lagoon, body (EH72)
- Tamago River, lower (EH81)
Predictions for the six model test sites. Shown is the site location, the group probability scores and the list of taxa predicted to occur (probability of occurrence in excess of 50%). Also shown are the taxa observed (*), the expected number of taxa and the observed to expected ratio of taxa.
Wingan Inlet, lower (EH07)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.02 | 0.06 | 0.00 | 0.00 | 0.12 | 0.80 |
Predicted taxa, in decreasing order of capture probability.
75% Limnoporeia kingi*
73% Nemertea unid.*
72% Gammaropsis (megamphopus) sp.*
70% Armandia MOV sp. 282*
55% Paracalliope lowryi
52% Nassarius jonasi*
51% Pseudogobius olorum
Predicted taxa = 7
Expected no. taxa = 4.49 *Observed no. taxa = 5
Ratio of Observed : Expected = 1.11
Merriman Creek, upper (EH18)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.61 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 |
Predicted taxa, in decreasing order of probability of capture.
72% Gammaropsis (megamphopus) sp.
68% Nemertea unid.
67% Simplisetia aequisetis*
60% Arthritica helmsi*
59% Pseudogobius olorum*
57% Amarinus laevis*
55% Nassarius jonasi*
54% Paracalliope australis
52% Dimorphostylis colefaxi*
52% Limnoporeia kingi
50% Nephtys australiensis
Predicted taxa = 11
Expected no. taxa = 6.45 *Observed no. taxa = 6
Ratio of Observed : Expected = 0.93
Lake Tyers, mid (EH29)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.03 | 0.00 | 0.96 | 0.00 | 0.01 | 0.00 |
Predicted taxa, in decreasing order of probability of capture.
99% Simplisetia aequisetis*
98% Melita festiva*
98% Paracalliope australis*
86% Tanytarsini spp.*
86% Urocampus carinirostris*
77% Macrobrachium intermedium*
67% Nemertea unid.*
66% Amarinus laevis
66% Ascorhis tasmanica*
66% Gammaropsis (megamphopus) sp.*
66% Pseudogobius olorum
65% Limnoporeia kingi*
65% Paracorophium excavatum
65% Philypnodon spp.*
64% Platyhelminthes unid.
64% Armandia MOV sp. 282*
56% Arthritica helmsi*
54% Capitella MOV sp. 2558*
Predicted taxa = 18
Expected no. taxa = 13.08 *Observed no. taxa = 14
Ratio of Observed : Expected = 1.07
Lake Yambuk, upper (EH53)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.87 | 0.00 | 0.01 | 0.04 | 0.00 | 0.07 |
Predicted taxa, in decreasing order of probability of capture.
93% Simplisetia aequisetis*
76% Arthritica helmsi*
72% Amarinus laevis*
69% Dimorphostylis colefaxi*
69% Gammaropsis (megamphopus) sp.*
63% Nemertea unid.*
63% Pseudogobius olorum*
61% Paracalliope australis*
59% Nephtys australiensis
56% Nassarius jonasi
55% Ascorhis tasmanica*
Predicted taxa = 11
Expected no. taxa = 7.35 *Observed no. taxa = 9
Ratio of Observed : Expected = 1.22
Curalo Lagoon, body (EH72)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.97 |
Predicted taxa, in decreasing order of probability of capture.
87% Limnoporeia kingi*
85% Armandia MOV sp. 282
75% Gammaropsis (megamphopus) sp.*
75% Nemertea unid.*
62% Paracalliope lowryi*
51% Paracorophium excavatum
50% Haplostylus dakini
50% Nassarius jonasi
50% Notospisula trigonella
50% Pseudogobius olorum
50% Sabellidae unid.*
Predicted taxa = 11
Expected no. taxa = 6.83 *Observed no. taxa = 5
Ratio of Observed : Expected = 0.73
Tamago River, lower (EH81)
Classification groups predicted from MDA.
1 | 2 | 3 | 4 | 5 | 6 |
0.02 | 0.00 | 0.00 | 0.00 | 0.62 | 0.35 |
Predicted taxa, in decreasing order of probability of capture.
67% Nemertea unid.
58% Nassarius jonasi*
58% Pseudogobius olorum
57% Macrobrachium intermedium
57% Nephtys australiensis*
52% Barantolla lepte
52% Gammaropsis (megamphopus) sp.*
Predicted taxa = 7
Expected no. taxa = 4.01 *Observed no. taxa = 3
Ratio of Observed : Expected = 0.75
Appendix 4: k-dominance curves
- K-dominance curves for dredge samples
- K-dominance curves for grab samples
A.4.1 K-dominance curves for grab samples, ordered in increasing community complexity.
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 1 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 1 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 1 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 1 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 1 Continued (below).
 Original Size of Above Image
Original Size of Above Image
K-dominance curves for dredge samples, ordered in increasing community complexity.
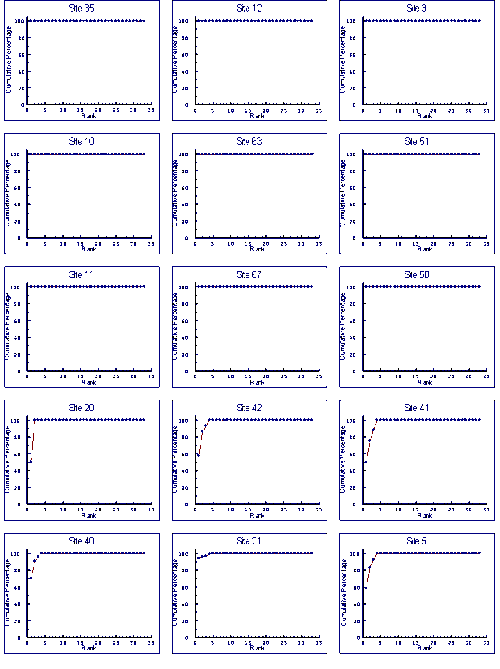
Appendix 4 Fig 2 K-dominance curves for dredge samples
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 2 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 2 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 2 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 2 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Appendix 4 Fig 2 Continued (below).
 Original Size of Above Image
Original Size of Above Image
Meta Data Record: Estuarine Health Assessment Using Benthic Macrofauna
<TITLE>Estuarine Health Assessment Using Benthic Macrofauna</TITLE>
<META NAME="DC.Title" CONTENT="Estuarine Health Assessment Using Benthic Macrofauna">
<META NAME="DC.Creator" CONTENT="John Moverley & Alastair Hirst, Museum Victoria">
<META NAME="DC.Type" CONTENT="text">
<META NAME="DC.Date" CONTENT="1999">
<META NAME="DC.Format" CONTENT="text/html">
<META NAME="DC.Coverage" CONTENT="Australia">
<META NAME="DC.Description" CONTENT="RIVPACS (River Invertebrate Prediction and Classification System) models were developed in the early 1980s to assess river health in the United Kingdom (Moss et al. 1987). As well as the United Kingdom, RIVPACS-type models (AusRivAS) are now being used throughout Australia for river health assessment. The major objective of this project was to evaluate whether a RIVPACS-type approach could be used to assess estuarine environmental health in Australia. This required two questions to be answered, can RIVPACS-type models be constructed for Australian estuarine habitats and, if they can, are these of value in assessing environmental health. A secondary objective was to evaluate other approaches to estuarine health assessment. This study investigated macrobenthic communities of coastal plain estuaries, whether the mouths were open or closed. Included were a number of brackish coastal lagoons that were intermittently open to the ocean and had small streams flowing into them.">
<META NAME="DC.Relation" CONTENT="This report describes the outcomes of a research project conducted under the Urban Research and Development sub-program of the National River Health Program (NRHP). The NRHP is an on-going national program established in 1993, managed by the Land and Water Resources Research and Development Corporation (LWRRDC) and Environment Australia. Its mission is to improve the management of Australia's rivers and floodplains for their long-term health and ecological sustainability">
<META NAME="DC.Source" CONTENT="Environment Australia community Information Unit">
<META NAME="DC.Subject" CONTENT="Water, rivers">
<META NAME="DC.Publisher" CONTENT="Land and Water Resources Research and Development Corporation. GPO Box 2182 Canberra ACT 2601">
<META NAME="DC.Publisher" CONTENT="Published Electronically on au.riversinfo.org by the Environmental Information Association (Incorporated) with the permission of LWRRDC and Environment Australia.">
<META NAME="DC.Rights" CONTENT="Copyright (©) LWRRDC">
<META NAME="DC.Identifier" CONTENT="Estuarine Health Assessment Using Benthic Macrofauna. John Moverley & Alastair Hirst, Museum Victoria. LWRRDC Occasional Paper 18/99 (Urban Subprogram, Report #11) 1999.">
<META NAME="DC.Identifier" CONTENT="http://au.riversinfo.org/library/nrhp">
<meta name= "DC.Language" content = "en">
 Riversinfo Australia (au.riversinfo.org): Publication Information:
Riversinfo Australia (au.riversinfo.org): Publication Information:
- Webserver publication date (version): Saturday, April 20th 2019.
- The publication of material on au.riversinfo.org was supported by the Commonwealth Government via the National Landcare Program and the National River Health Program.
- End users are advised to expect variations in formatting and layout depending on both the format of the original material, as supplied by the authors, and the end users particular software and hardware configuration(s).
- In the case of variation between this document and a hard copy original the original takes precedence.
- Except when otherwise stated, in writing, information provided by au.riversifno.org is provided "as is" without warranty of any kind to anyone. The entire risk as to use of the information rests with the user. Should the information prove defective, the user of the information assumes the cost of all necessary repair or correction. In no event, unless agreed to in writing, will anyone associated with au.riversinfo.org be liable for any damages arising out of the use or inability to use the information, even if au.riversinfo.org has been advised of the possibility of such damages.
- As of January 2004 au.riversinfo.org is archived under Precision Info.
Copyright ©
- Authors and the public are reminded that the purpose of au.riversinfo.org is to compile and reference Internet information about rivers in an easily accessible and comprehensive manner to assist all those seeking to preserve and publicize Australia's Rivers.
- au.riversinfo.org has no interest in acquiring or assuming exclusive copyright over any authors material published on au.riversinfo.org. The copyright of material appearing on au.riversinfo.org rests with existing copyright holders who give approval for publication on au.riversinfo.org.
- The copyright status of articles is generally stated within the article and those seeking to reproduce the article further should, if required, seek permission from the copyright holder prior to any further publication or circulation.
- Where no explicit copyright statement has been made copyright will be assumed to be held by the author of the article.
- au.riversinfo.org principally converts and marks up existing material from various formats. The au.riversinfo.org content delivery scripts, mark up code and presentation are copyright.
- Consistent with the aims stated in point 1 and provided you do not attempt to substantially reproduce large sections of the au.riversinfo.org Internet site you may, provided you have the permission of the copyright holders, copy and distribute verbatim copies of the files appearing on au.riversinfo.org provided that you conspicuously and appropriately publish on each copy an appropriate notice acknowledging the source of the material.
- Consistent with the aims stated in point 1 and provided you do not attempt to substantially reproduce large sections of the au.riversinfo.org Internet site you may, provided you have the permission of the copyright holders, copy and distribute modified versions of the files appearing on au.riversinfo.org provided that you conspicuously and appropriately publish on each copy an appropriate notice acknowledging the source of the material and stating the date and nature of any changes you may have made to the material.
- If you systematically copy and or re-distribute information (or modified information) originating from au.riversinfo.org then you must:
- Accompany the distribution with a written offer to transfer the information to any third party, wishing to use it in a manner consistent with the aims stated in point 1, a complete machine-readable and interchangeable copy of the corresponding material.
- Any charges to the third party arising as a result of the transfer must not exceed the cost of your physically performing the transfer.
- The offer must be valid for at least three years from the date of your last systematic distribution of the material.
- Acceptable machine readable and interchangeable file formats include .html and or .txt and or .rtf formats. PDF format is NOT considered an acceptable machine interchangeable file format.
- You must cause any work that you distribute or publish, that in whole or in part contains information derived from au.riversinfo.org, to be available under the principles of these same distribution terms
- Third parties receiving material deriving from au.riversinfo.org should be advised that they will be bound by the principles of these distribution terms
Acknowledgments
Current at December 2002
 au.riversinfo.org has been developed to facilitate public access to scientific and technical information on Australia's Rivers. Much of this material was paid for using public funds and is therefore owned by the Australian people. Environment Australia have been instrumental in making available all the publications from:
au.riversinfo.org has been developed to facilitate public access to scientific and technical information on Australia's Rivers. Much of this material was paid for using public funds and is therefore owned by the Australian people. Environment Australia have been instrumental in making available all the publications from:
- The National River Health Program
- The Ausrivas Assessment Scheme
 The au.riversinfo.org Internet site is in-part supported by the Natural Heritage Trust. The Natural Heritage Trust focuses on five key environmental themes - land, vegetation, rivers, coasts and marine, and biodiversity. The programs of the Natural Heritage Trust play a major role in developing sustainable agriculture and natural resource management, as well as protecting our unique biodiversity through improved management and delivery of resources.
The au.riversinfo.org Internet site is in-part supported by the Natural Heritage Trust. The Natural Heritage Trust focuses on five key environmental themes - land, vegetation, rivers, coasts and marine, and biodiversity. The programs of the Natural Heritage Trust play a major role in developing sustainable agriculture and natural resource management, as well as protecting our unique biodiversity through improved management and delivery of resources.
 The au.riversinfo.org Internet site is in-part supported by Sydney Airport who have co-funded a Natural Heritage Trust supported project intended to develop Internet based environmental information integration and management systems. This will assist in developing an overall perspective on the demands and uses for environmental resources
The au.riversinfo.org Internet site is in-part supported by Sydney Airport who have co-funded a Natural Heritage Trust supported project intended to develop Internet based environmental information integration and management systems. This will assist in developing an overall perspective on the demands and uses for environmental resources






















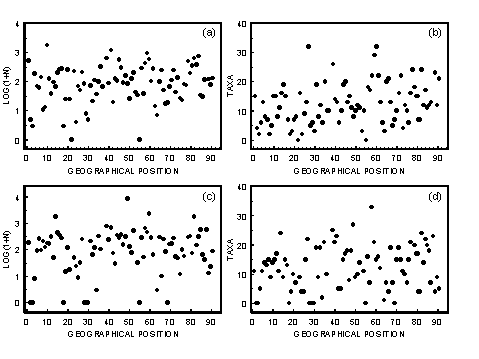
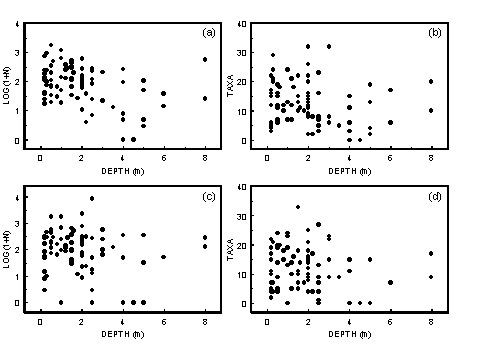
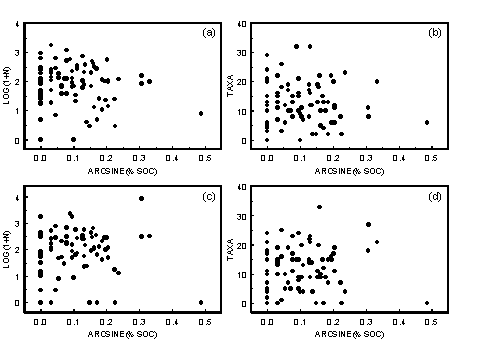
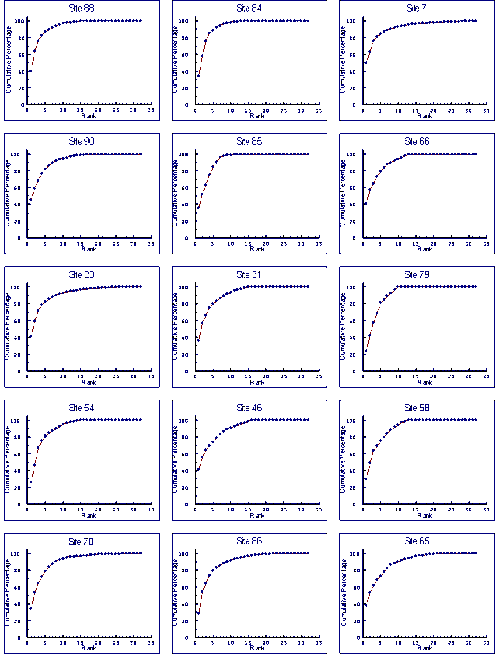
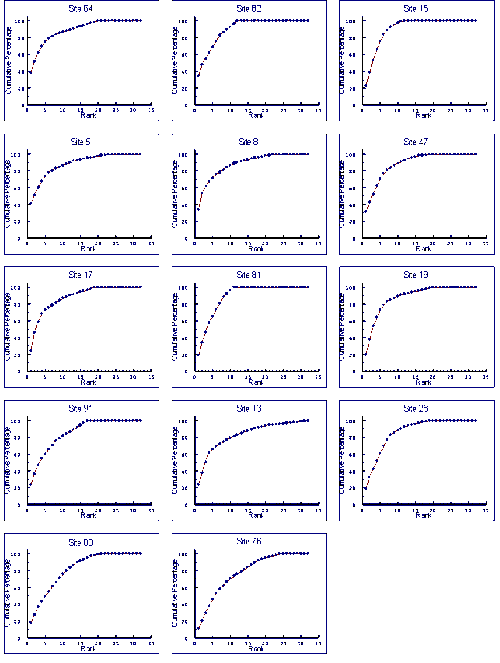

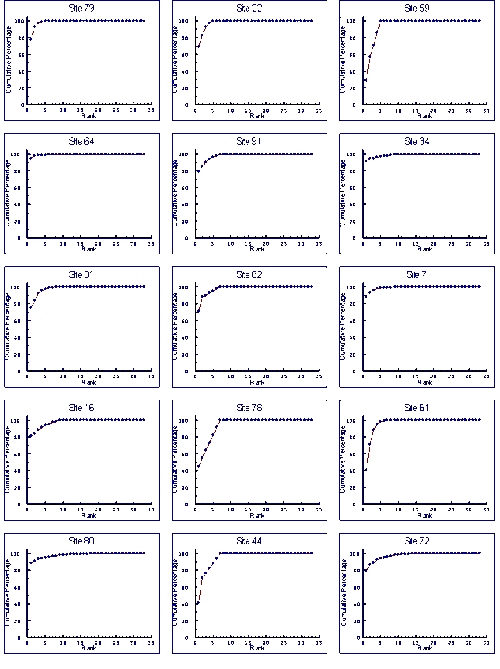
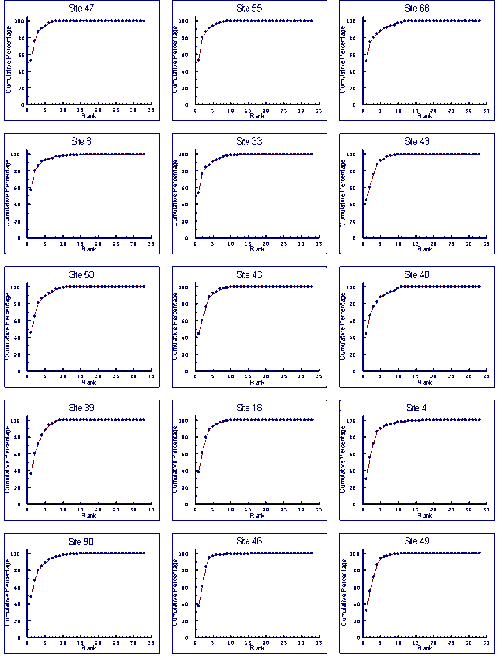
 Riversinfo Australia (au.riversinfo.org): Publication Information:
Riversinfo Australia (au.riversinfo.org): Publication Information:


