As of January 2004 au.riversinfo.org is archived under Precision Info
RIVERSINFO AUSTRALIA ARCHIVE
This archive provides non stylised versions of selected reference documents (formerly) resident on au.riversinfo.org.
D.M. Deeley and E.I. Paling
Marine and Freshwater Research Laboratory
Institute for Environmental Science Murdoch University
LWRRDC Occasional Paper 17/99 (Urban Subprogram, Report No. 10)
December 1999
Foreword, Publication Information & Acknowledgements
2. Factors influencing the distribution of estuarine biota
3. Distribution of biota in estuaries
4. Impact of urban and rural development on the distribution of estuarine biota
5. Ecological health assessment in estuaries
6. Utility of biota for indicating the ecological health of estuaries
No single environmental indicator will unambiguously define the interactions between ecosystem form and function, resilience and stability of biological communities and response of the estuarine system to anthropogenic stress. It is necessary to evaluate a broad range of potential measures simultaneously, in order to define appropriate ecological health indicators to underpin the management effort. There is however, no certainty in the selection and evaluation process and even with the best efforts, type I (false positive) and type II (false negative) errors are likely and both may prove expensive. Increased confidence in the selected indicator suite can flow from an evaluation of the monotonicity of correlated indicators, especially when assessments show consistent patterns arising from physico-chemical measures and measures of biotic community structure for various trophic groups.
Physico-chemical indicators of ecosystem processes have provided reliable information in the past, but problems have arisen from attempts to relate these measures to biological endpoints, particularly for estuaries with large interannual variability. In the absence of biological data for estuarine ecosystems experiencing extreme heterogeneity of climatic influence, such as estuaries in the cyclone belt, physico-chemical indicators, or socio-economic indicators of anthropogenic influence may be the only option. Paleolimnological investigations may also provide additional insight, but the degree of taxonomic resolution required and the cost of stable isotope analysis may require considerable resources.
An evaluation of available historical data can better define temporal and spatial heterogeneity of systems and define normal behavior and natural variability. Iterative refinement of the optimum indicator suite will require considerable ongoing research, monitoring and evaluation. Unfortunately, estuaries by their nature are 'slow systems' with decadal time constants for iterative loops of management measures and assessment of their success. It is a relatively simpler task to define indicators, which describe the status quo (e. g. degree of eutrophication), but it is much more difficult to develop a predictive capacity.
Autotrophic protistans (periphyton, phytoplankton), appear to be useful for describing nutrient enrichment, salinity and pH profiles, but complicating factors such as the nature of coupling of secondary predation need to be identified. Autecology of local indicator species also needs to be defined. Zooplankton appear to be limited as environmental indicators, but may be useful as elements of biotic indices across trophic groups. One of the major impediments to using planktonic organisms for inferring the condition of estuarine health is the considerable vertical, horizontal and temporal heterogeneity displayed by these organisms in both disturbed and undisturbed systems.
More recently, benthic macro-invertebrates have been successfully used to describe the nature and magnitude of organic enrichment of estuaries. Community structure, biomass and relative abundance of functional groups and indicator species have also been developed and used as environmental indicators.
Measures of community structure have problems because of a lack of information about interactions governing diversity and evenness of biotic communities and stability and resilience of the ecosystem. Species richness, diversity indices and measures of biomass have probably been the most widely used indicators in the majority of published works, but generally without appropriate critical analysis of their utility.
A myriad of biotic indices (ratios of functional groups) within and across trophic levels have been described in the international literature. There are problems in defining weightings for elements contributing to biotic indices and the loss of valuable information during these types of data reduction limit their potential. Detailed autecology of members of functional groups are required for biotic indices and this type of information is potentially available for some cosmopolitan species, but generally lacking for endemic species which may describe important nuances of the local environment.
As with biotic indices, there is a range of combined metrics described in the literature. Metrics generally combine physico-chemical elements, and may include some biological information. Many of the problems with biotic indices apply equally to metrics, but when calibrated for a particular local situation, they offer considerable discriminatory power.
For Australian estuaries, physico-chemical measures of catchment and estuarine processes and socio-economic measures of anthropogenic influence may be of use. If assumptions about the linearity of interactions between the diversity of biotic communities and the stability and resilience of ecosystem function are valid, then conventional measures of community structure will also provide useful insights.
A hierarchy of environmental indicators is required for Australian estuaries, which provide for assessment of current status, a measure of diagnostic precision and a robust predictive capacity ('early warning'). Of the range of potential indicators evaluated in this review, some core indicators have been used successfully by managers, some will require further development and others will need considerable additional research before links between stress and response have been established.
The ongoing selection, evaluation and refinement of environmental indicators for assessing the ecological health of Australian estuaries, needs to proceed as a close partnership between land and waterway managers and scientific specialists.
This report describes the outcomes of a research project conducted under the Urban Research and Development sub-program of the National River Health Program (NRHP).
The NRHP is an on-going national program established in 1993, managed by the Land and Water Resources Research and Development Corporation (LWRRDC) and Environment Australia. Its mission is to improve the management of Australia's rivers and floodplains for their long-term health and ecological sustainability, through the following goals:
Urban streams and estuaries (i.e. those affected by runoff and discharges from urban areas) are an important subset of Australia's waterways. Most are degraded biologically, physically and chemically and therefore require appropriate methods to be developed for health assessment and management. The Urban R&D Sub-program, managed by the Water Services Association of Australia, comprises 8 research projects which were developed to meet research priorities for urban streams and estuaries within the goals of the NRHP and to complement existing NRHP projects on non-urban rivers. Thus, research focuses on development of standardised methods for assessing the ecological health of urban streams and estuaries which can be linked with data on water and sediment quality. The urban R&D projects commenced in 1996.
The definition of health in urban waterways used is "the ability to support and maintain a balanced, integrative, adaptive community of organisms having a species composition, diversity and functional organisation as comparable as practicable to that of natural habitats of the region".
The eight projects of the Urban Sub-Program are:
| Decision support system for management of urban streams | Dr John Anderson Southern Cross University, Lismore |
| RIVPACS (River InVertebrate Prediction and Classification System) for urban streams | Dr Peter Breen CRC for Freshwater Ecology, Monash University, Melbourne |
| DIPACS (Diatom Prediction and Classification System) for urban streams | Dr Jacob John
Curtin University, Perth |
| Sediment chemistry- macroinvertebrate fauna relationships in urban streams | Dr Nick O'Connor Water EcoScience, Melbourne |
| Classification of estuaries | Dr Peter Saenger Southern Cross University,Lismore |
| Literature review of ecological health assessment in estuaries | Mr David Deeley
Murdoch University, Perth |
| Estuarine health assessment using benthic macrofauna | Dr Gary Poore
Museum of Victoria, Melbourne |
| Estuarine eutrophication models | Dr John Parslow CSIRO Marine Laboratories, Hobart |
Assessing the ecological health of estuaries in Australia
D.M. Deeley and E.I. Paling
Marine and Freshwater Research Laboratory
Institute for Environmental Science Murdoch University
LWRRDC Occasional Paper 17/99 (Urban Subprogram, Report No. 10)
December 1999
ISSN: 1320-0992
ISBN: 0-642-26767-7
Cover Photograph: Wilson Inlet Western Australia, July 1996.
Published by:
Land and Water Resources Research and Development Corporation
GPO Box 2182 Canberra ACT 2601
Telephone: (02) 6257 3379
Facsimile: (02) 6257 3420
Email: public@Iwwrrdc.gov.au
WebSite: www.lwrrdc.gov.au
© LWRRDC
Published Electronically on au.riversinfo.org by the Environmental Information Association (Incorporated) with the permission of LWRRDC and Environment Australia. Environment Australia assisted by providing copies of the manuscript for electronic publication. The Natural Heritage Trust provided project funds which were used to assist in publishing this material. In the case of variation between this document and the hard copy original the original takes precedence. (Bryan Hall).
The information contained in this publication has been published by LWRRDC to assist public knowledge and discussion and to help improve the sustainable management of land, water and vegetation. Where technical information has been prepared by or contributed by authors external to the Corporation, readers should contact the author(s), and conduct their own enquiries, before making use of that information.
Funding for this investigation was provided by the Urban Water Research Association of Australia. Funds were also made available as part of the Estuarine Health Indicators Project which was jointly funded by the National Landcare Program through the National Heritage Trust, the Western Australian Water & Rivers Commission and Murdoch University.
The authors would like to acknowledge Kevin Bancroft for invaluable assistance with the literature search and construction of the bibliographic database.
Guidance for this review was provided by Prof. AJ McComb and Assoc. Prof. J John.
Review of an earlier draft of this document was provided by Prof. D Lord, Assoc. Prof. D Walker, Dr K Hillman, Dr D Hamilton, Dr G Douglas and Dr T Rose. Their comments are greatly appreciated.
For Yolande Ben and Rachel
Australia covers a wide range of geographical and climatic regions from the northern tropics to cool southern temperate regions and the Mediterranean climate of the southwest. There is considerable variation in the pattern of rainfall for estuarine catchments throughout Australia. As a consequence of interactions between rainfall and geomorphology of coastal areas, Australian estuaries display a wide diversity of forms across regions (Table 1. 1, Ferguson, 1996; Roy, 1996).
Estuaries by their nature are highly productive, heterogeneous systems and they usually have extensive communities of fringing, benthic and pelagic biota. Agricultural development throughout Australia and urbanization and industrial development on the coastal fringe, have resulted in increased loads of sediment, nutrients and other pollutants to estuaries which have lead to significant changes to many estuarine habitats (SOMER, 1995).
The nature of impacts caused by increased sediment and pollutant loads on biological communities in estuaries throughout Australia is not well understood, even though there have been numerous investigations in some estuaries, particularly those surrounded by major cities. This lack of system-wide understanding has arisen partly because of the dynamic nature of Australian estuaries and the requirement for extensive spatial and temporal data sets to describe 'natural' variability of physico-chemical and biological processes.
Agenda 21, the international action plan to achieve sustainability of natural resource management recognizes in its final chapter, the need for information to guide decision-makers. In conclusion, Agenda 21 calls for the development of indicators which can measure sustainability. Sustainable development in this context has been defined in much wider terms than the conservation of natural resources and focuses on the needs of people and of maintaining the quality of life. It is often considered that sustainable development needs to proceed as an integrated partnership between social, economic and natural resource managers (Midmore et al. , 1995). The OECD has developed environmental indicators, which assess environmental performance, integration of policies and environmental accounting. The 'pressure, state, response' model has been proposed and adopted widely (Midmore et al. , 1995). Pressure (or stressor) indicators describe the degree of human impact on receiving ecosystems. State indicators define the state of the system or its degree of exposure to stressors, and response indicators define the nature of policy and management instruments used to mitigate pressures on ecosystems (Midmore et al. , 1995). The Australian and New Zealand Environment and Conservation Council has adopted the OECD model with minor changes (ANZECC, 1998).
The Australian national strategy for ecologically sustainable development has as a core objective "to protect biological diversity and maintain essential ecological processes and life support systems in marine and estuarine waters" (Governments, 1992; SOMER, 1995). The pressure, state, response framework adopted by ANZECC has been used as a basis for developing a set of core indicators for assessing the long term performance of receiving waterways in Australia and New Zealand (Fairweather and Napier, 1998; Ward et al. , 1998a; Ward et al. , 1998b). The Australian national system for State of Environment (SOE) reporting was established as part of the National Strategy for Ecologically Sustainable Development (Governments, 1992) and seeks to provide key environmental indicators.
'Core' environmental indicators for estuaries can be defined as that minimum set of indicators, which when properly monitored, will provide information on major trends and impacts on estuarine ecosystems. There must be sufficient background knowledge for core environmental indicators in order to describe departures from normal behavior within natural variability and to provide an understanding of the range of natural biophysical processes and relationships contributing to the ecological health of Australian estuaries. Environmental indicators used to describe pressures on estuaries and aspects of estuarine health are summarized in Table 1. 2. This report describes the application of some of these indicators.
| Table 1. 1 Distribution of estuary types in Australia (After, Ferguson, 1996). | ||||||
|---|---|---|---|---|---|---|
| Region | Regional area km2 |
Annual rainfall mm |
Runoff mm | Tidal range m | Dominant estuarine morphotype | Seasonal mixing regimes |
| Timor Sea | 539,000 | 600 - 1,200 Summer maximum | 138 | 2. 0 - 10. 5 | Macrotidal drowned river valleys, Mature | DRY inverse estuary WET short lived fresh flush |
| Gulf of Carpentaria | 640,800 | 600 - 1,600 Summer maximum | 99 | 2. 2 - 7. 7 | Macrotidal drowned river valleys, Mature | DRY inverse estuary WET short lived fresh flush may occur |
| Northeast Coast | 454,000 | 800 - 4,000 Summer maximum | 183 | 1. 6 - 6. 3 | Barrier, Drowned river | DRY well mixed WET highly stratified during high discharge |
| Southeast Coast | 268,000 | 600 - 1,600 Year round | 136 | 1. 1 - 1. 8 | Barrier, Drowned river | DRY well mixed, WET highly stratified during high discharge |
| Tasmania | 68,400 | 600 - 3,200 Year round | 69 | 0. 9- 2. 5 | Drowned river | DRY salt wedge, WET salt wedge |
| South Australian Coast | 75,730 | 300 - 800 Winter maximum | 7 | 0. 5 - 2. 0 | Barrier | no data available |
| Southwest Coast | 140,000 | 400 - 1200 Winter maximum | 52 | 0. 4 | Barrier | DRY highly stratified, increasingly saline WET freshwater flush |
| Indian Ocean | 520,000 | 200 - 400 Arid | 12 | 0. 5 - 5. 8 | Drowned river | DRY permanent inverse circulation WET short lived freshwater flush |
| Table 1. 2 Environmental indicators used to define ecosystem health of estuaries. | |||
|---|---|---|---|
| Component | Item | Environmental indicator | Pressure/ State / Response |
| Ecosystem | Communities | stability | S |
| diversity | S | ||
| productivity | S | ||
| Catchment | Attributes | drainage density | S |
| erosion | P | ||
| landuse | P | ||
| land management practices | R | ||
| flood frequency, peak flow velocities | S, P | ||
| runoff quality (nutrients, sediment) | S, P | ||
| nutrient pollution index | S | ||
| stream width-depth ratio | S | ||
| vegetation status, catchment, riparian zones | S | ||
| Waterbody | Attributes | dissolved concentration potential | S |
| equilibrium discharge | S | ||
| flushing (retention time) | S | ||
| stratification | S, P | ||
| particle retention efficiency | S | ||
| Biotic condition | General | mixed-function oxidase (fish biomarkers) | S |
| presence of 'abnormal' organisms, attributes | S | ||
| Pelagic | phytoplankton composition, chlor-a, biomass, cell size | S | |
| biota | periphyton composition, biomass, autotrophic index | S | |
| macrophyte community composition, biomass | S | ||
| translocated species | S | ||
| fish community composition, biomass | S | ||
| Benthic biota | benthic community composition, biomass | S | |
| benthic community indices | S | ||
| Habitat | Water quality | biochemical oxygen demand (BOD) | S |
| clarity, turbidity (Secchi depth) | S | ||
| Chlorophyll-a | S | ||
| pH | S | ||
| P, N and toxicant concentrations,N:P ratios | S | ||
| salinity, conductivity | S | ||
| silica concentrations | S | ||
| temperature | S | ||
| water depth, levels | S | ||
| combined water quality index | S | ||
| Sediments | organic matter content | S | |
| percent silt-clay | S | ||
| sediment nutrient concentrations | S | ||
| dissolved oxygen concentrations | S | ||
(Table 1. 2 - After Rochford, 1951; NOAA, 1988; NOAA, 1989; Alve, 1991; Riding, 1992; Ahl, 1993; Cairns Jr. et al. , 1993; NOAA, 1993; Harris, 1994; Harris, 1995; Holdway et al. , 1995; Strobel et al. , 1995; ANZECC, 1998; Fairweather and Napier, 1998; Ward et al. , 1998a; Ward et al. , 1998b)
Ecological health can be defined as the maintenance of the structural and functional attributes including natural variability and succession, of a particular ecosystem (Cairns Jr. , 1993a), or the absence of ecosystem distress syndrome (Haskell et al. , 1992). The term ecosystem distress syndrome has now become well established in the literature and some of the attributes of estuaries displaying ecosystem distress syndrome are summarized in Table 1. 3.
Composite measurement endpoints that define ecosystem health must include assessment of ecosystem response to stress. Biotic measurement endpoints commonly used as environmental indicators of health may include species richness and abundance, dominance of preferred species, functional group distributions, condition of organisms, loss of functional redundancy, loss of homeostatic self-regulating properties and loss of productive capacity (Cairns Jr. and Niederlehner, 1992).
The homeostatic ability of mammals is considerably different to that of ecosystems. Ecosystems are not programmed by genetic codes reflecting evolutionary fitness, but reach points of equilibria determined by interactions between their biotic and abiotic components (Wicklum and Davies, 1995). If viewed at larger temporal scales, ecosystems are constantly evolving and may only reach quasi-equilibrium for relatively short periods of geological time.
System scale measures of health or integrity need to consider the development and maintenance of spatial heterogeneity, the spatial and temporal interactions and exchanges across heterogeneous landscapes, the influences of heterogeneity on biotic and abiotic processes and the management of heterogeneity (Cairns Jr. and Niederlehner, 1992). Because of the spatial patterning of landscapes, flows and transfers between spatial components assume special importance and the process of redistribution of organisms, materials and/or energy among landscape components is considered an essential feature of landscape ecology (Cairns Jr. and Niederlehner, 1992).
| Table 1. 3 Symptoms of ecosystem distress syndrome described in the literature. | |
|---|---|
| Impact | Observation |
| Changes in nutrient capital | Changes in runoff, estuarine waters, sediments and biota |
| Changes in primary productivity | Macrophytes and/or phytoplankton |
| Simplification of food webs | Loss of mutualisms other complex behaviors, shorter food chains |
| Changes in patterns of resource utilization | Usually reduced efficiency of utilization |
| Reductions in species diversity | Single groups or over several trophic groups |
| Increased dominance by opportunists | R-strategists increase in dominance |
| Reductions in size distribution of biota | Loss of larger, longer lived organisms |
| Increased amplitude of species populations | Boom and bust population dynamics |
| Increased prevalence of disease, abnormalities | Caused by xenobiotic influences, increased stress |
| Circulation of contaminants in biota and media | Xenobiotic influences |
(Table 1. 3 - (After Havens 1994, Birkett and Rapport 1996, Nielsen and Jernakoff 1996, Odum 1985).
Ecological health may be considered a multidimensional concept that cannot be easily measured or monitored. This theoretical construct is based on interpretations of measures of stocks and flows of the various biotic components and their abiotic environment (Amir and Hyman, 1993). The appropriateness of the concept of ecosystem health has resulted in considerable debate in the literature (Amir and Hyman, 1993; Cairns Jr. , 1993a; Cairns Jr. , 1993b; Suter, 1993; Cairns Jr. , 1994; Shrader-Frechette, 1994; Wicklum and Davies, 1995).
Scientists do agree, however, on the need for measures of anthropogenic stress and ecosystem resilience or susceptibility to stress. A recent statement by the Union of concerned Scientists in a "World Scientists' Warning to Humanity 1992," stated that human beings are so altering the natural world, that it may eventually be unable to sustain life (pp 456 Shrader-Frechette, 1994). Clearly there is a need for an ecological 'Canary in the Cage' that is suitable for use at the ecosystem level because such alterations without appropriate measures of change cannot be controlled. It is therefore essential to have operational definitions of ecosystem health that can be applied to management problems.
Ecosystems by nature are dynamic and all have various degrees of ability to resist external pressures ('resistance') and to return to 'normal' states following perturbation ('resilience' or 'stability' Harris, 1994). Characteristics correlated to ecosystem health include biomass, productivity, nutrient cycling, species richness and diversity, food web complexity, niche specialization, spatial diversity, size distribution of organisms and their life styles, disease prevalence and mortality rates (Amir and Hyman, 1993). However, these indicators by themselves may not show the true integrated nature of a healthy ecosystem (Wicklum and Davies, 1995). This is because all these characteristics are data-dependant and attempt to describe complex systems.
It has been argued that rarer species increase an ecosystems ability to respond to unusual events (Pielou, 1975). The populations of a community may therefore be divided into two components; structure and inventories. Structure reflects the majority of organisms that contribute to productivity, whereas inventory includes rarer species which may contribute to redundancy or resilience of the ecosystem.
Health is therefore a delicate balance between structure and function within the two extremes of orderly versus unpredictable perturbation. Ecosystem-wide turnover times are influenced by structural and functional complexity. Long turnover times may characterize an ecosystem rich in diversity, biomass, redundancy and relatively lower productivity (Amir and Hyman, 1993). Conversely, shorter turnover times may characterize ecosystems that have received anthropogenic stresses. A central theme of this review is that there are numerous exceptions to these general rules however, and some ecosystem processes may have very short turnover times even where there is no evidence of anthropogenic disturbance. It is argued that this is especially true for ecosystems subjected to significant climatic variability, such as Australian estuaries.
Ecosystem health has been compared to the human view of our own state of well being. Human health may be defined on many scales including, the ability to maintain a high state of self organization and regulation, the maintenance of motor and intellectual skills, availability of good food and shelter, the ability to reproduce, the ability and opportunity to communicate, possessing spiritual well-being, or having a sense of making a useful contribution to society. Many of these broad definitions of different aspects of human health are highly subjective and not readily measured in absolute terms. Like human health, the concept of ecosystem health appears also to be highly subjective (Wicklum and Davies, 1995).
Human health may be viewed at a variety of scales, all of which provide different information. Organ or cellular health does not generally reflect the health of other organs or cells or of the individual as a whole. Healthy cancer cells, for example, may be leading to the death of the individual. Community health of humans is generally assessed at larger spatial and temporal scales. Health statisticians commonly look at regional populations and compare trends in disease occurrence in the population over lengthy time spans, reinforced by many years of accumulated medical experience.
For the individual, health practitioners use blood pressure and vital signs as integrating surrogates for human health. Here they use a simple measure to gain an insight into the state of an extremely complex organism that may by influenced by a wide range of stressors (diseases). In some instances, blood pressure and vital signs do not correlate with ill health, and medical practitioners need to use additional tests to confirm worrisome symptoms.
There are clearly some parallels that can be drawn from the human health metaphor when applied to ecosystems. Some similarities between ecosystem and humans are:
Like human health, total ecosystem health is not easily measured, but there are measures that correlate to health. Ecosystem health, like human health, can be viewed at a variety of spatial and temporal scales depending on the required outcomes. Usually ecosystem ill health is more easily defined than health.
Accordingly, no single measure or group of measures will provide a perfect view of the stability and resilience of an ecosystem, and inconsistencies may regularly confound health assessments (Fairweather, 1997). It is often simpler to identify a stressed ecosystem on the verge of becoming disintegrated than to pinpoint clear and prevalent signs of its integrity and health (Amir and Hyman, 1993). Consequently, it may only be possible to undertake a system performance audit, or to assess the success of the chosen integrating measures of health (Bailey et al. , 1992), over decades, or after considerable monitoring and refinement of the ecosystem measures.
Critics of the concept of ecosystem health or integrity (Suter, 1993; Shrader-Frechette, 1994; Wicklum and Davies, 1995), correctly raise concerns about the mindless acceptance of inappropriate metaphors, simple measures of complex dynamic systems. It has been argued that people must value nature from a variety of temporal and spatial scales and from a multiplicity of perspectives. Proponents of the concept of ecosystem health argue that non-technical managers require readily understood measures in order to promote public and political concern over the degraded state of many of our estuarine ecosystems (SOMER, 1995).
Just as the Dow-Jones measure of economic performance is a simple indicator of complex national and international economic transactions, health indicators also tend to integrate and simplify. The act of integration and simplification loses scientific information in favour of human value judgments. It has been recommended that a range of indicators of different trophic levels and physico-chemical interactions be evaluated simultaneously, and iteratively refined for particular situations (Amir and Hyman, 1993; Cairns Jr. et al. , 1993).
It can be concluded that even though there are problems with the concept of ecological health through the over-simplification of complex multi-dimensional problems, the potential tradeoffs arising from the accessibility of the concept to the wider community renders it a useful concept. A diagnosis of ill health would certainly raise community and political concern.
None could argue that there is a degree of urgency accompanying the need for the evaluation and testing of potential environmental indicators to assess the ecological health of Australian estuaries. However, care needs to be taken in their development and application.
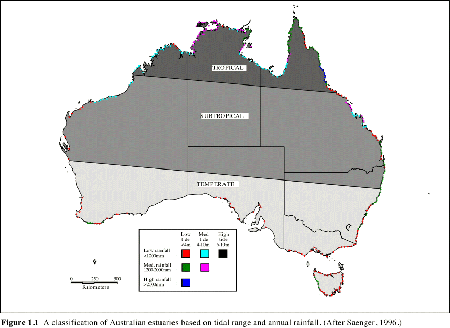
Because of the great variability of physical, chemical and biological characteristics of Australian estuaries, no two systems are exactly alike. It is possible however to identify general properties which help classify estuaries. Estuaries have been described as drowned river valleys, barrier estuaries, saline coastal lagoons and mature riverine estuaries, or whether they posses salt wedges, are stratified, reverse or well mixed. The nature of tides, rainfall, evaporation and river inputs to estuaries have a major influence on salinity regimes and ultimately biological communities. Estuaries in the northwest, eastern coast and southeast, although having varying tidal amplitude (Figure 1. 1), generally show strong diurnal tidal signals (Figure 1. 2). Estuaries in the southwest have restricted tidal amplitude together with a semi-diurnal tidal signal. This has resulted in very low levels of marine flushing in southwest estuaries.
It has also been observed (Jennings and Bird, 1967; Bayly, 1975), that estuarine entrances from Shark Bay in Western Australia around the southern sea board to Fraser Island in Queensland are subjected to persistent high energy wave action. As a consequence, barriers have formed at estuarine mouths which restrict marine incursion into estuaries. Estuaries around the tropical northern coasts of Australia are subjected to less persistent wave action are of lower energy and are characterized by wide-open estuaries, which often have protruding deltas. These properties are reinforced by the differences in tidal range (Figure 1. 2).
Northern Australia receives rainfall in summer with little winter rain (Figure 1. 3). The rainfall patterns for much of the eastern coast of Australia are more consistent throughout the year, with smaller differences between average winter and summer rainfall. The southwest of Australia experiences a Mediterranean climate with most rainfall falling in winter.
The rivers and streams which deliver runoff to Australian estuaries, show pronounced inter-annual variability (Alexandra and Eyre, 1993) and very large floods relative to normal discharge patterns. The coefficient of variation (standard deviation/mean) of Australian river flow (CV = 0. 85), is considerably greater than that of Northern American (CV = 0. 36) and European (CV = 0. 28) rivers (Eyre and Twigg, 1996).
For extreme events, the difference between Australian rivers and those elsewhere in the world becomes even more pronounced. The 100 year recurrence interval flood(Q100) divided by the mean annual flood (Q) for Australian rivers (5. 9) is greater than that for American and European (3. 5 and 2. 2 respectively) rivers (Finlayson and McMahon, 1988). This is caused partly by rainfall and evaporation patterns in a normally very dry continent and because of catchment antecedent wetness conditions following unseasonal rains. Extreme flow events may not come from extreme rainfall events, but rather follow normal amounts of rainfall on a previously wetted catchment.
There are clearly other major factors which influence the character of estuaries, and a number of estuarine classification schemes have been described in the literature. These include those based on morphology which have used characteristics such as; genesis and evolution, morphology, relief, catchment geology, sediment dynamics, tides, wave climate and barrier dynamics, (Pritchard, 1967; Hume and Herdendorf, 1988; Reddering, 1988; Cooper and Ramm, 1994). Other schemes based upon hydrological and circulation patterns have used; runoff inputs, tidal range, tidal prism, stratification and mixing zones (Pritchard, 1967; Heath, 1975; Hume and Herdendorf, 1993 Alexander and Monaco, 1994).
 |
| Figure 1.1 A classification of Australian estuaries based on tidal range and annual rainfall. (After Saenger, 1996. ) |
| (original size image) |
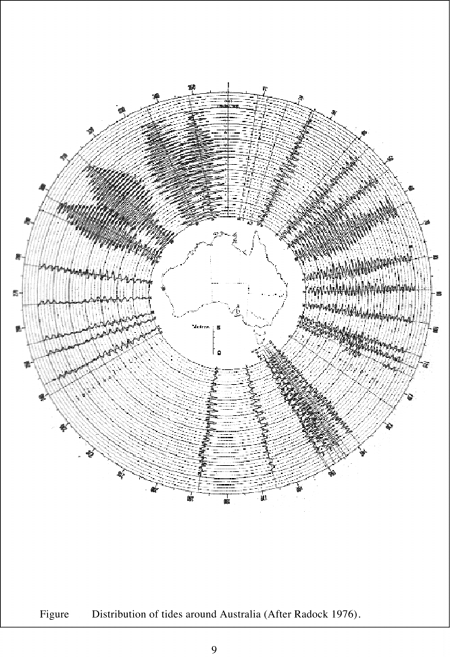 |
| Figure 1.2 Distribution of tides around Australia (After Radock, 1976). |
| (original size image) |
Classification schemes which use a combination of morphology, hydrology and biological characteristics(Dethier, 1992; Bucher and Saenger, 1993) have also been described in the literature, and in addition to using those characteristics described above, have also used substratum type, fringing communities and major animal and plant groups to classify estuaries.
It has been argued (Roy, 1996), that climate, estuarine geology, hydrology and biology form a hierarchical succession, in that the underlying geology of the catchment and estuary define the basin and entrance conditions, which in turn influence the salinity and mixing regime, which in turn influence biotic communities. These together with climate, have a major influence on the biological communities supported by estuaries. When the dynamic nature of recent sea level changes are also considered, it is not surprising to find significant departures from generalized schemes applied to the various regions.
The interactions between climate, estuarine geology and hydrology and their impacts on the nature of biological communities are discussed in later chapters of this report.
The aim of this report has been to compile a review of literature on estuarine benthic macrofauna, benthic algae and plankton in relation to health assessment and related topics in Australia. Specifically:
The assessment of ecological health and the distribution of estuarine organisms in space and time are controlled by physical, chemical and biological processes in estuaries under the influence of natural seasonal cycles. Thus the following structure has been used to focus on these processes.
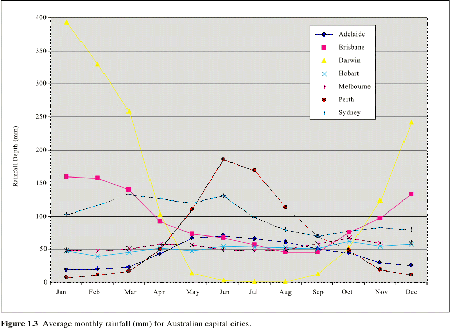 |
| Figure 1.3 Average monthly rainfall (mm) for Australian capital cities. |
| (original size image) |
Within each section throughout the review general features of a topic are explored followed by specific examples which illustrate agreement with, or departure from accepted relationships. Concluding statements are provided at the end of each section.
This review was required to describe literature on estuarine benthic macrofauna, benthic algae and plankton in relation to health assessment and related topics in Australia. It was found that there were some gaps in the Australian estuarine literature describing particular interactions for some trophic groups and for some pollutant impacts and accordingly, key international publications were reviewed where required. Additionally, for regions where there was not an extensive estuarine literature, such as the northern tropics and for South Australia, information from local marine embayments or freshwater systems was used to illustrate particular points.
A central theme for this review is that Australian estuaries are so highly variable in space and time, that processes and relationships established for estuaries subjected to less climatic variability elsewhere in the world, may not apply here. All environmental indicators tend to simplify complex dynamic processes and interactions and thus require careful consideration if meaningful conclusions about changes to ecosystem health are to be made, particularly where some degree of early warning is required. It is argued that seasonal variability in rainfall, runoff and salinity, and the natural patterns of distribution and abundance of Australian estuarine biota make it difficult to distinguish background behavior from human induced perturbations. Therefore, the use of Australian estuarine biota to describe the early onset of changes in ecosystem health may not be without considerable difficulty, particularly for diffuse-source inputs of sediment, organic material and nutrients.
Estuaries are dynamic systems with cycles and processes operating at a range of spatial and temporal scales. The dynamic nature of estuarine processes, coupled with natural cycles of disturbance together with anthropogenic stressors, act to distribute estuarine biotic communities into spatial and temporal mosaics. Factors influencing the distribution of estuarine organisms include; wave action, cyclones, runoff, salinity, nutrients, turbidity, light, linkages between other trophic groups and substratum characteristics, (Furnas, 1995; Keough and Butler, 1995). These general rules may not apply in all situations however, because seasonal variations in phytoplankton community structure and biomass, have been observed where salinity, temperature and nutrient concentrations, have marked seasonal patterns (Harris et al., 1987). By way of contrast, in some areas, such as the Gulf of Carpentaria, there may not be a marked seasonality in salinity, temperature and nutrients, and consequently little apparent seasonality in phytoplankton biomass (Burford et al., 1995).
It has often been difficult to isolate the influence of anthropogenic stressors on biotic communities where there has been a relatively poor understanding of natural cycles of disturbance, reproduction, migration, colonization and predation. As an example, natural factors which have the potential to deplete seagrass beds include; disease, climatic changes, storms and cyclones, sediment movement, salinity changes, sea level changes and faunal influences (Larkum and West, 1982). These natural disturbance factors in seagrass communities may be compounded by anthropogenic stressors such as; nutrient enrichment, increased shading by epiphytes, increased turbidity and detrital fallout from sewage outfalls, reduced light levels and thermal plumes from power stations. Causal links between anthropogenic stressors and adverse impacts, may be somewhat easier to define where the impact has been severe, or in close proximity to the source of disturbance. It has been much more difficult to ascribe causality where the source of the impacts is remote from the disturbances, where the impacts have been less severe, or where there has been significant natural disturbance.
This section briefly discusses physical and chemical conditions influencing the distribution and abundance of biota in Australian estuaries not subjected to human disturbance. This section describes the normal range of conditions observed in estuaries, together with the impact of episodic natural disturbances, which are an important feature of Australian estuaries.
The underlying geology, evolutionary history and climate influence the nature of estuaries through their control of pedogenesis and catchment hydrological regimes and ultimately on estuarine basin morphology (Roy, 1996) and these factors are discussed below.
Inherited factors such as bedrock type, coastal morphology and geology control the size and shape of estuarine basins and the nature of sediment supplied to them. Contemporary processes such as tidal currents, river discharge and ocean waves also influence the modes of sedimentation in estuaries and estuary hydrodynamics. Regional climates including factors such as temperature and rainfall - runoff regimes directly affect estuarine salinities, nutrient cycling and ecological conditions (Roy, 1996).
The inherited coastal morphology that was inundated at the end of the Postglacial transgression also plays an important role in governing the morphology of estuarine basins. Coastal types may be simply categorised into two classes; low relief, gently sloping coastal plain coasts and more rugged and embayed, bedrock controlled coasts. Estuaries on low relief gently sloping coasts tend to be shallower than those on rugged, incised coastlines (Roy, 1996).
Catchment pedogenesis also plays an important role in controlling the nature of sediments delivered to estuaries. Granite rocks produce a sandier stream bedload than do basalts. Catchments with fine textured soils produce stream bedloads rich in clay and silt sized particles. The nature of sediments delivered to and stored within estuaries, influences both nutrient storage and regeneration and the form of benthic communities.
The stage of estuarine evolution also governs the morphology of estuaries. One general aspect of estuarine evolution is a progressive reduction in water area (Roy, 1996). Most of the infilling occurring during estuarine maturation results from fluvial sedimentation, the incorporation of biogenic material in the estuary and in cases where there is significant longshore movement of sediment, from the building of flood-tide deltas. Rates of infilling are governed by the rates of sediment delivery to the estuary and by the nature of tidal currents. As the estuary infills and shallows, smoother sedimentary shorelines develop and there is a greater proportion of soft-bottom sediments throughout the estuarine basins. Rates of mud accretion range from 0.15-1.0 mm yr-1 in slow filling estuaries, to 5 mm yr-1 in faster filling estuaries (Roy, 1996).
The delivery of fluvial sediment to estuaries varies with river flow, with floods delivering proportionately greater sediment loads. It was estimated that more than 100,000 t of sediment were delivered to the Beaufort estuary in Western Australia, in a single flood event. This equates to around 12 mm of sediment added to the 7 km2 estuary over several days (Hodgkin and Clark, 1988).There has been a significant increase in sediment delivery to estuaries following clearing for agriculture, and while soil erosion is obvious throughout agricultural areas, sedimentation of rivers and estuaries is much less apparent (Hodgkin and Clark, 1988).
The impact of sedimentation and sediment types on estuarine biota is discussed in Sections 4.3.2 and 4.6.2.
Topographic features of the estuarine basin and entrance channels may modify currents, restrict mixing and lead to the development of stratification and unusual chemical environments such as anoxia, which influence nutrient relationships (D'Adamo et al., 1992).Classification of Australian estuaries (Ferguson, 1996; Roy, 1996), may include (Table 1.1); drowned river valleys, barrier estuaries, saline coastal lagoons and mature riverine estuaries. Drowned river valleys are typically deep, narrow with steep rocky sides and with sand bodies associated with tidal deltas, which may extend from the open mouth into the lower estuary. These estuaries may demonstrate vertical and horizontal salinity stratification during periods of higher river flow.
Barrier estuaries range in size and may be very large. The Peel-Harvey estuary, a barrier estuary in Western Australia, has an open water area in excess of 145 km2. Barrier estuaries are characterised by having long narrow entrance channels, which restrict tidal currents, or sand bars at the mouth, which close periodically. Wind-driven circulation is usually more important than tidal currents in mixing estuarine waters in barrier estuaries.
Saline coastal lagoons are generally small features located in coastal valleys with smaller catchments. Saline coastal lagoons in earlier stages of their evolution may have resembled barrier estuaries. River inputs are accommodated by evaporation and percolation through porous sand bars at the mouth. Sand bars at the mouth may breach on occasions, allowing the influx of marine water.In arid environments or Mediterranean regions with an absence of summer rainfall, hypersaline conditions may develop in estuaries.
Mature riverine estuaries are narrow, permanently open channels where there is significant river flow for much of the year combined with meso or macro-tidal influences. These estuaries are restricted to the lower reaches of mature river valleys with low relief and normally have meandering channels. Sediments lining the river channel are usually fine-grained silts and clays.
In the absence of river flows, the total volume of water moving into and out of an estuary with each tidal cycle, the tidal prism, is dependant on the dimensions of the estuary and the tidal range. It is also affected by lesser factors such as tidal amplification or attenuation, the lag phase and tidal resonance (Ketchum, 1983).
Solar radiation is the basis of photosynthetic activity for all autotrophic organisms in estuaries including phytoplankton, benthic and attached algae, macroalgae, seagrasses and fringing vegetation communities. Material produced as a result of photosynthesis forms the basis of all estuarine food webs. As an example of the importance of light in maintaining entire aquatic ecosystems, nearly half the animal species associated with seagrass communities were found to have declined in mesocosms placed in darkness (Edgar, 1990b).
Aquatic species have evolved with particular optimum temperatures for growth and reproduction. Temperature may influence rates of respiration and photosynthesis in aquatic organisms as well as other enzymatic activities and growth processes. Temperature may also effect the water column directly, especially in deeper waters where temperature dependant changes in density may lead to stratification of the water body. Hypolimnetic oxygen depletion has been observed in thermally stratified water bodies (Bernhardt and Clasen, 1985). Higher water temperatures mean lower levels of dissolved oxygen and hypolimnetic oxygen depletion resulting from stratification may be more likely at higher temperatures. Temperature was found to have an influence on the abundance of benthic macro-invertebrates in the Hawkesbury estuary (Jones, 1987), and water temperature was suggested to have influenced the collapse of a Nodularia bloom in Orielton Lagoon, Tasmania (Jones et al., 1994).
Given different temperature tolerances and optima for various estuarine organisms, it is not unexpected that literature reports on the effects of temperature on estuarine organisms are ambiguous. Temperature distribution was found to have a significant influence on benthic microalgal production (Rasmussen et al., 1983) and on the distribution of zooplankton communities in the Brisbane River estuary, and was thought to be as important as salinity in imposing stress on brackish water copepods (Bayly, 1965). Conversely, only a minor part in the annual variation of phytoplankton production in Port Hacking appeared to be related to temperature, but the nature of the relationship was uncertain (Scott, 1979).
In many situations it may be difficult to separate the influence of temperature from other factors such as salinity, turbidity and light. Species diversity was found to be lower during winter-spring for diatom communities in the Swan River estuary, but this may have been caused by a combination of lower temperatures, salinities and light levels (John, 1988). Temperature had little effect on fish densities in a tropical estuary in northern Australia, but turbidity and salinity were inversely related to fish numbers (Cyrus and Blaber, 1992. Neither temperature nor turbidity were correlated with catch rates of the most abundant fish species in three Queensland estuaries (Sheaves, 1998).
The impact of thermal pollution on estuarine biota is discussed in Section 4.4.2.
Light penetration in estuaries has a significant influence on the nature of pelagic and benthic plant communities (Scott, 1979; McComb and Lukatelich, 1986) and may be influenced by the colour of the water, turbidity, depth and angles of incidence. Light attenuation is generally greater for estuaries than for nearshore marine areas because of greater levels of turbidity(Scott, 1978). Turbidity levels in estuarine waters are influenced by living and dead organic particles and suspended sediments. There may be marked seasonal influences on turbidity fields in estuaries as river discharges vary seasonally. Resuspension of bottom sediments has also been found to greatly increase sediment-water column nutrient exchange and affect the forms and amounts of productivity in estuaries (Gabrielson and Lukatelich, 1985).
Water transparency may be influenced by initial chlorophyll-aand suspended solids concentrations, and the interactions of settling and dilution by tidal exchange (Scott, 1978) and by the presence of natural organic matter, tannins and mineral colloids (Wrigley et al., 1988). Different spectral distributions have been observed for estuarine, coastal and oceanic waters. Maximum transmittance for estuarine waters may occur at around 575 nm compared to 465 in clearer oceanic systems (Smith and Baker, 1981).
The relationship between light penetration and primary production has been established to the point that significant correlations between water clarity and seagrass depth penetration have been used as a biological indicator of light availability (Abal and Dennison, 1996). A gradient of increasing turbidity and suspended solids moving upstream from the mouth of the Brisbane River, was found to influence seagrass distribution (Abal and Dennison, 1996). Annual variation in primary production in Port Hacking estuary was found to be related to solar irradiance (Scott, 1978), and average annual light attenuation was linearly related to average annual biomass of macroalgae in a shallow temperate estuary (Lavery et al., 1991).
Light availability may not have the same influence on benthic microalgae as on other aquatic plants. For example, it has been found that benthic microalgae can maintain a high production even at irradiances less than 1% of water-surface PAR (Shaffer and Sullivan, 1988). Light availability was found to influence the abundance benthic microalgae in marine systems while nutrient levels influenced biomass (Masini, 1990).
The relationship between light levels and secondary production is not clear, but may depend on the availability of food for primary consumers to pass to higher order consumers and on predation. For example, light levels were found to have influenced species assemblages in copepods, but not for nematodes (Austen, 1989). The distribution patterns of zooplankton in coastal embayments provided evidence that visual predation was greater in shallow waters and had resulted in the exclusion of a species vulnerable to visual predators (Kimmerer and McKinnon, 1989). This may mean that lower light levels may cause reduced phytoplankton production hence food supply for primary consumers, but also provide a lower risk of predation by visual predators.
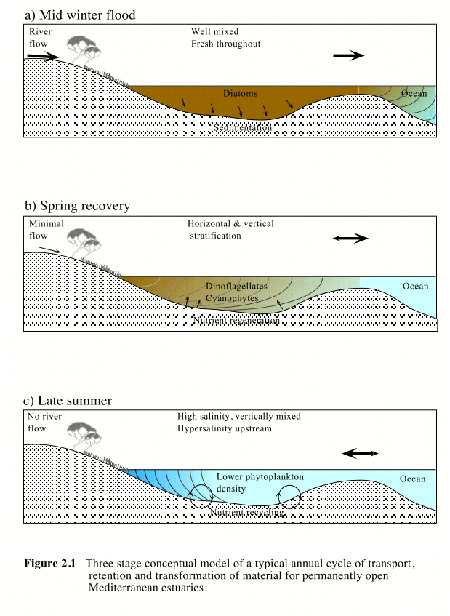 |
Figure 2.1 Three stage conceptual model of typical annual cycles of transport, retention and transformation of material in permanently open Mediterranean estuaries. |
| (original size image) |
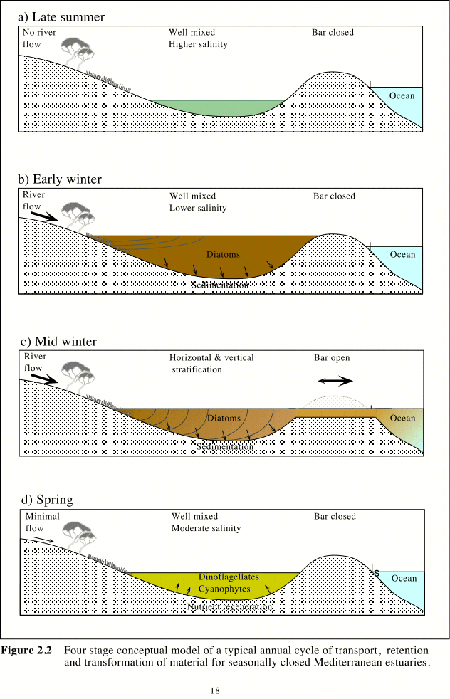 |
Figure 2.2 Four stage conceptual model of a typical annual cycle of transport, retention and transformation of material for seasonally closed Mediterranean estuaries. |
| (original size image) |
There was a well-defined negative relationship between macrobenthic production and tannin-stained river water in the Bathurst Channel estuary in southwestern Tasmania (Edgar, 1991). Macrobenthic production was an order of magnitude lower at sites with poor light penetration compared to sites with clearer marine water in the downstream section of the estuary. The reduction of macrobenthic production in the presence of tannin-staining of the water may have related to reduced primary production where light penetration was less and reduced food availability for the macrobenthos.
It may be concluded that light availability has a direct relationship on primary production for a range of estuarine macrophytes and phytoplankton and that secondary production may be influenced indirectly by light levels through food and habitat availability. The relationship between light and visual predation may however influence the community composition of secondary producers. The impact of pollutant inputs and light reductions on estuarine biota is discussed in Section 4.6.2.
Estuarine water balances are dominated by freshwater inputs from stormwater runoff, groundwater, direct rainfall onto the estuary surface, evaporation and marine exchange. There is considerable seasonal variability in each of these fluxes particularly under Australian conditions (Section 1.3). The following sections discuss aspects of the estuarine water balance which influence the distribution of estuarine biota.
River flows are the main source of freshwater, sediment, nutrients and silica for estuaries although in some situations smaller, but significant levels of sediment and nutrients may enter estuaries from adjacent marine areas (Eyre and France, 1997). For the northern and eastern coasts of Australia, much of the rainfall and streamflow occurs over summer (Eyre, 1994; Eyre and Twigg, 1996; Eyre and Twigg, 1997) whereas for the southwest of WA, the saline phase occurs during summer (Chalmer et al., 1976; Congdon and McComb, 1979).
Estuaries on the northern and eastern coasts of Australia have been described as having three distinct phases. A fresh phase follows very heavy rainfall, when flood flows completely expel saline water, bypassing many estuarine processes and depositing sediment, nutrients and biota into nearshore waters. During the fresh phase, hydraulic residence times or estuarine flushing times may be reduced to days, under the influence of strong river currents. The estuaries may be well mixed with particulate and dissolved constituents showing little vertical and horizontal structure. Clearly the complete expulsion of saline water from an estuary is one extreme of a continuum which is controlled by the interactions of basin morphology and depth, river flows and tidal dynamics. In some estuaries, complete expulsion of saline water may occur several times each year, while in other estuaries, this may never occur, or only under extreme events.
The fresh phase of an estuary is followed by a recovery phase where the saline waters penetrate into the estuary under tidal influence. The saline intrusion may lead to stratified conditions as denser marine waters underlie less dense surface waters. Wind driven currents and tidal actions mean that stratified conditions may occur only for a short period during the recovery phase. In some estuaries, constricted entrances and narrow shallow estuarine reaches may cause stratified conditions to persist for weeks or months (Stephens and Imberger, 1996). During the recovery phase, dissolved and particulate constituents may establish complex vertical and horizontal gradients.
The third phase or the normal condition (i.e. that which occurs for much of the time), is where a horizontal salinity gradient is established under normal river flow regimes. The dynamics of dissolved constituents in estuaries under various streamflow regimes are discussed in Section 2.4.
Estuaries in the southwest of Australia also have distinct phases, depending on whether they are permanently open or seasonally closed. For permanently open estuaries under a Mediterranean climatic regime (Figure 2. 1), the three phases are similar to those observed in eastern coast estuaries except that the flood phase occurs in winter. The spring recovery phase is usually accompanied by horizontal and vertical salinity stratification. In eutrophic estuaries, salinity stratification may lead to anoxic conditions accompanied by significant nutrient regeneration and phytoplankton blooms.
In summer, the normal pattern is minimal or no river flow and permanently open estuaries become fully marine. Hypersaline conditions may develop upstream in some estuarine reaches remote from marine influences. These reverse estuaries provide particular challenges to estuarine fauna, which may be exposed to both fresh and hypersaline conditions within the same year.
For seasonally closed estuaries under a Mediterranean climatic regime (Figure 2. 2) there are four distinct phases. The first phase is in late summer where water levels are at a minimum and salinities are at a maximum following evaporative losses over summer. Estuaries of this type in arid environments may develop significant hypersalinity at these times. Stokes Inlet, near Esperance in Western Australia, has salinities approaching 65 in later summer.
The second phase is in early winter following the onset of rainfall and river flows. Water levels in the estuaries rise to above sea levels and estuarine salinities fall. Water levels in the estuary may increase by over 4 m from the summer minimum to the early winter maximum. During this phase, all runoff together with dissolved and suspended materials are retained within the estuary. Estuarine water levels continue to rise with continued river inputs until the sand barrier at the mouth breaches. Following breaching of the bar, water levels fall rapidly over several days until estuarine water levels approximate those of the sea. It is during this third phase when marine water enters the estuary under tidal influence immediately following the breaching of the bar, that significant vertical and horizontal salinity stratification establishes. Limited marine exchange may continue for some time while river flows continue to keep the sand bar open. It is during this time that recruitment of estuarine biota from nearshore marine areas occurs.
Nearshore sand movement under storm induced wave action and reduced winter river flows cause the sand bar at the mouth to close at the end of winter. The fourth, or spring phase normally sees salinity stratification broken down a short time after bar closure under the influence of wind driven circulation and mixing. It is during this phase that the closed estuary resembles a saline coastal lagoon. In eutrophic estuaries, nutrient regeneration mechanisms may lead to significant blooms of phytoplankton following bar closure in spring.
The concentrations of dissolved constituents in estuaries are far more variable than oceanic waters, varying temporally; daily, seasonally, inter-annually and spatially; with increasing distance from the mouth, with depth and laterally. The following sections describe the patterns of dissolved constituents in Australians estuaries that influence the distribution of estuarine biota.
Horizontal and vertical salinity gradients in estuaries are a result of interactions between the total volume of the estuary, inputs of river water and seawater, precipitation and evaporation, tidal mixing, currents and topography of the estuarine basin. Salinities in estuaries are normally between 0 and 35, although for some estuarine reaches subjected to saline river inputs or very high net evaporation rates in summer, hypersaline conditions may develop with salinities exceeding 50.
There may be vertical and horizontal salinity gradients in estuaries seasonally depending on the relative influences of tidal currents and river flows. Salt wedge estuaries are dominated by river flow and may have a lens of fresh water extending over more dense marine waters for much of the length of the estuary. Changing river flows adjusts the relative position of the upstream limit of the marine water in the estuary. These estuaries are typical for much of Europe and America where there is less variability between periods of higher and lower river flows.
Estuaries with greater tidal influence and a greater width to depth at the mouth, normally show greater advective and diffusive mixing of fresh and marine waters forming a significant zone of intermediate salinities. Tidal currents may be significantly greater than river flow in setting up a two layered system of currents. Flood tides have greatest velocities at depth and ebb tides have greater velocities at the surface (Adam et al., 1992).
Estuarine organisms have evolved to take advantage of tidal currents to maintain their position within an estuary against a net seaward current. There may also be a lateral salinity gradient in these estuaries as freshwater outflows are influenced by the earth's coriolis effect (Pritchard 1955 cited Biggs and Cronin, 1979). In estuaries where tidal currents are stronger relative to river flows, longitudinal and lateral advection and lateral diffusion become dominant and the vertical salinity stratification is lost.
The capacity of water to dissolve oxygen is significantly reduced with increasing salinity and temperature. An increase in salinity of approximately 10, which occurs with the initial transition of the saline wedge in a salt-wedge estuary, reduces the oxygen carrying capacity of the water by approximately 20%. This has important implications for the distribution of estuarine biota.
The complex patterns of salinity in estuaries have a profound influence on the distribution of estuarine organisms. Various classification schemes have used salinity as a surrogate for the distribution of estuarine organisms. For example, the bottom salinity isohaline of 2, has been suggested as a simple habitat indicator of declining freshwater inflows to the San Francisco Bay estuary. This isohaline is associated with the turbidity maxima and is correlated with the abundances of a range of benthic and pelagic organisms from various trophic groups (Jassby et al., 1995).
Nutrient cycling is critical to the higher biological productivity of estuaries when compared to marine waters. The following section discusses the dynamics of nutrient inputs, losses and regeneration within estuaries.
Nitrogen, phosphorus and silica have been considered to be the main nutrients required for plant growth in estuaries (Kennish, 1994). The concentrations of these three major elements are typically greater in estuaries than in the open ocean. Runoff is the main source of macro elements although the atmosphere and marine waters may also contribute minor amounts (Eyre and France, 1997).
The three primary dissolved forms of N are ammonium (NH4-N), nitrite (NO2-N) and nitrate (NO3-N), although dissolved organic and particulate forms also comprise a significant N source. Ratios of inorganic constituents and oxidised inorganic N to particulate organic N ratios, are also influenced by dissolved oxygen concentrations (Balls et al., 1996).
Phosphorus is an essential component of the adenosine triphosphate-diphosphate energy transferring mechanism and of nucleic acids. In aerobic natural waters, P occurs as the oxidised state HPO42-, with minor amounts of PO43-, H2PO4-, as a constituent of organic compounds, or adsorbed onto mineral particles. In natural waters, concentrations of P are normally low, especially in very oligotrophic waters.
Silica is required to build the skeletons of diatoms and other protistans, and because of ready biological uptake of this element, its concentrations are generally spatially and temporally variable. Particulate forms of Si include fine quartz and clay minerals and although present in well-mixed estuarine waters, these forms are not readily available for plant growth because of their very low solubilities.
The concentrations of N, P and Si, are strongly influenced by riverine and groundwater inputs and bio-chemical processes in estuaries. For example, there was considerable variability in the concentrations of NO3, PO4 and Si in the Port Hacking estuary throughout the year (Scott, 1979). Surface nitrate concentrations in the Swan River estuary were significantly correlated with rainfall, which delivered the nutrients in shallow groundwater (Thompson and Hosja, 1996), or from surface runoff from the urbanised catchment.
Nutrient behavior during post flood recovery of the Richmond River Estuary, NSW (Eyre and Twigg, 1997), is typical of many Australian estuaries and is discussed here as an example of estuarine nutrient behavior (Figure 2.3). During the flood phase, Si, NO3, TN, DIP and TP were found to be at higher concentrations at the head of the estuary with lowest salinities and decreasing approximately linearly downstream, as dilution and mixing with marine waters increased. Dissolved oxygen and pH were lower at the head of the estuary, reflecting the influence of lower pH runoff containing refractory organic matter.
During the recovery phase, dissolved and particulate constituents were found to establish complex vertical and horizontal gradients. Ammonium concentrations were found to increase downstream from the head of the estuary. This was suggested to have been a result of decomposition of particulate N from the sediments which flocculated from flood waters as they met marine conditions during the flood phase (Eyre and Twigg, 1997). Silica, NO3 and TN did not vary greatly with salinity. There was a small decrease in TP downstream.
Dissolved inorganic P had the highest concentrations at the head of the estuary with rapidly decreasing concentrations moving downstream, as biological processes and adsorption reactions at lower pH values (< 7.4), remove dissolved P. Moving further downstream (as salinities rose) the pH increased (8.2), and an increase in dissolved P was observed. This was thought to be a result of desorption of P from mineral oxides at higher pH values (Eyre and Twigg, 1997).
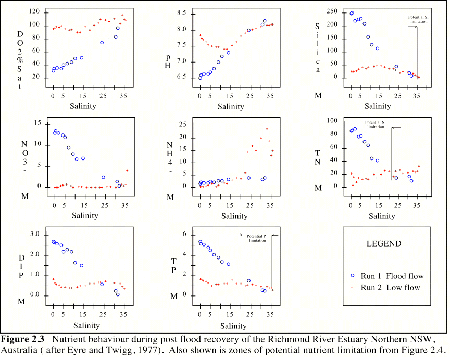 |
Figure 2.3 Nutrient behavior during post flood recovery of the Richmond River Estuary Northern NSW, Australia (after Eyre and Twigg, 1977).Also shown are zones of potential nutrient limitation from Figure 2.4. |
| (original size image) |
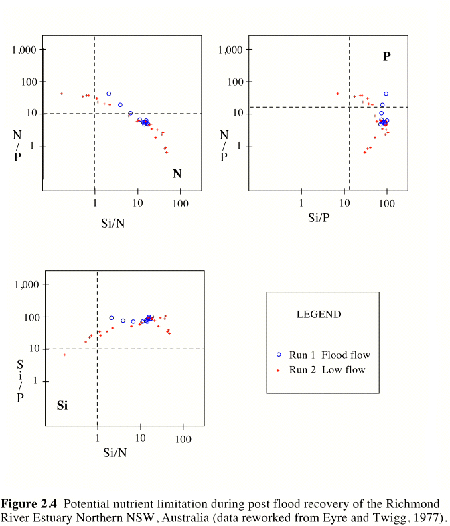 |
Figure 2.4 Potential nutrient limitation during post flood recovery of the Richmond River Estuary Northern NSW, Australia (data reworked from Eyre and Twigg, 1977). |
| (original size image) |
The above patterns are similar to those observed for Mediterranean estuaries with respect to streamflow, except that runoff events occur in winter (Thompson and Hosja, 1996). In summer, nutrient levels in Mediterranean estuaries with permanent openings approximate those of the ocean with a net gain of nutrients to the estuary over summer. There are usually very high concentrations of nutrients in these estuaries over winter (Thompson and Hosja, 1996) accompanied by a large net loss of nutrients to the ocean (Black et al., 1981).
The potential for nutrient limitation may impact the nature of plant communities in estuaries. There has been considerable discussion on the potential for nutrient limitation in marine and estuarine waters (Howarth, 1988). Nitrogen may be the main limiting nutrient in many estuaries, although P and Si have also been found to be limiting in some situations. In the Swan River estuary over summer, bioassays indicated that the available pool of N was up to 20 times more limiting to biomass development than was available P (Thompson and Hosja, 1996). For sediments, the nature of limiting nutrients may be more complex than that observed for overlying waters. For example, experiments with seagrasses found that P rather than N, was the primary limiting nutrient in marine carbonate sediments (Short et al., 1990), but the reverse was the case for terrigenous sediments.
A reworking of the nutrient data for the Richmond River described above, shows the typical pattern of potential nutrient limitation in estuaries (Figure 2.4. Samples results falling within the quadrants in each graph marked N, P or Si, identify the potential for nutrient limitation. Samples with potential nutrient limitation identified in Figure 2.4 were then superimposed onto the data for TN, TP and Si in Figure 2.3. This shows that during flood and recovery phases there was potential for N limitation where salinities were less than 20. In the estuary where salinities ranged from 20 to 35 there was the potential for P limitation, and at the mouth of the estuary during the recovery phase, there was potential for Si limitation. These patterns of potential N limitation in fresh to brackish waters, potential P limitation in saline to marine waters and potential Si limitation in marine waters were consistent with published accounts of nutrient behavior in other estuaries (Howarth, 1988).
The ratio of macro-nutrients in estuary has the potential to impact on community structure of phytoplankton. When the ratio of N:P falls below the Redfield ratio (Redfield, 1958), organisms that are able to fix atmospheric N are favoured. The ability of organisms to fix atmospheric N influences the abundance of various estuarine autotrophs. Some cyanobacteria under favorable conditions have the ability to fix atmospheric N through biochemical activity in their heterocycts (Ressom et al., 1993) and thus outcompete other species.
The concentrations of minor and trace elements present in estuaries are usually low, but these elements are essential for the growth and reproduction of many estuarine organisms. Dissolved and particulate organic material may interact and alter the concentrations of minor elements through chelation and complexing. Sediments may play an important role in helping to maintain the concentrations of minor elements in estuarine waters through adsorption/desorbtion reactions, dissolution and precipitation and biological processes. Changes in redox potential or pH can promote chemical reactions that mobilize minor elements and change their concentrations in interstitial and overlying waters. Organisms play an important role in transporting and accumulating minor and trace elements.
Trace elements in natural systems are delivered to estuaries in river flow as either dissolved or particulate fractions. Trace metals originate in parent rock material and soils following biochemical and weathering processes on the catchment. Trace metals may be carried to estuaries as various forms including: in solution as inorganic ions and both organic and inorganic complexes; adsorbed onto surfaces; in solid organic particles; in coatings on detrital particles after co-precipitation with and adsorption onto iron and manganese oxides; in lattice positions of detrital crystalline material; and precipitated as pure phases, possibly on detrital particles. The estuarine environment and biogeochemical processes control the partitioning and availability of metals (Kennish, 1991).
The availability of metals in estuarine waters, may be influenced by temperature, salinity and the presence of organic compounds, with which the metals form complexes. The bioavailability of metals may be very different to the total metal content. Research has revealed that dry weight metal concentrations that elicit toxicity can vary by one or two orders of magnitude among different sediments (Di Toro et al., 1990), and even when concentrations of metals had substantially exceeded background levels, metal bioavailability was found to be minimal (Ankley et al., 1996a). Bioavalibility of metals in sediments has been shown to be related more to interstitial porewater concentrations than to dry weight metal concentrations and that acid volatile sulfide is a key partitioning phase controlling cationic metal activity and toxicity (Ankley et al., 1996b).
In a pristine estuary (Bathurst Harbour, Tasmania), concentrations of dissolved cadmium and nickel were low and were essentially the same as total reactive concentrations. Copper and zinc on the other hand, had a greater association with particles, as the dissolved fractions were often less than the total reactive concentrations. Concentrations of copper, cadmium and nickel were comparable with those of open-ocean seawater. Total reactive concentrations of manganese and iron were high in river water entering the harbour, but these fell as salinity increased probably caused by complexing with organic particles. The very high concentrations of organic matter draining in to the harbour in river flow played a significant role in the availability of metal species (Mackey et al., 1996).
The concentrations of trace metals may be influenced by physical and biological processes. For example, particulate concentrations of Pb, Zn and Cd were found to be influenced by resuspension of metal-rich sediments and by uptake of dissolved metals by unicellular algae (Ferguson, 1983). Bivalve molluscs in Shark Bay, Western Australia, were found to have body burdens of cadmium in excess of 10 mg kg-1, compared to the limit of 2. 0 mg kg-1 (Australian food standard A12), even though these molluscs were remote from any known anthropogenic inputs. Studies revealed that cadmium in waters adsorbed extremely efficiently onto the surface of iron oxides, a common constituent of suspended solids in the region. In the molluscan digestive tract, the lower pH reversed the sorption reactions, allowing the cadmium to be accumulated by the animals. This means that these levels of cadmium accumulation were probably natural in origin (McConchie and Lawrance, 1991).
Dissolved gasses exist in estuarine waters, sediments and interstitial waters. The concentrations of dissolved gasses are a function of salinity, temperature, partial pressure of atmospheric gasses, dynamics and the air/water interface and biochemical processes in estuarine waters and sediments. Gaseous exchange at the air/water interface may be enhanced under windy conditions, when surface films are broken down.
Estuaries receive dissolved oxygen (DO) from atmospheric replenishment across the air-water interface and during photosynthesis by autotrophic organisms. Dissolved oxygen is lost from estuarine waters through respiration by organisms and through oxidation of organic materials in waters and sediments. The concentrations of DO in estuarine waters are influenced by the partial pressure of atmospheric oxygen, oxygen differential at the air-water interface, temperature, salinity, photosynthetic and respiratory rates of organisms and sediment and water column biochemical oxygen demand.
Dissolved oxygen and carbon dioxide are of fundamental importance to estuarine organisms because of their role in photosynthesis and respiration. Biological activity strongly influences the concentrations of these gasses in estuarine sediments and waters.
Sediments having a high biochemical oxygen demand and a finite DO supply may develop anoxic conditions. For example, sediment dissolved oxygen concentrations, were found to have fallen to less than 1 mg l-1, after seven days of still conditions in the eutrophic Peel-Harvey estuary, Western Australia and were accompanied by redox potentials of -200 mV, PO4-P concentrations of 60 µg l-1 and NH4-N concentrations of 800 µg l-1 (Lavery et al., 1991). It was suggested that in the still conditions of summer that this mechanism may provide a source of nutrients to dense algal banks supplementing reserves stored over winter.
Carbon dioxide is the main source of carbon used by photosynthetic organisms to manufacture more complex carbohydrates. Carbon dioxide is part of the CO2-carbonate-bicarbonate chemical system that buffers estuarine waters against rapid changes in pH. The modulating influence of CO2 in the estuarine environment plays an important role in maintaining many of the biogeochemical processes in estuaries. The concentration of CO2 in estuaries influences the rate of photosynthesis.
Spatial and temporal heterogeneity of estuarine constituents influences the distribution of biota, particularly for Australian estuaries. The nature of hydrodynamic process such as circulation and mixing may have a profound influence on the survival of particular biotic communities. For example, it has been shown (Ketchum, 1954) that the rate of circulation in an estuary may determine the minimum rate of reproduction required to maintain an endemic population of neutrally buoyant passive organisms. Consequently a species of this type which is able to maintain a population in one estuary may be unable to do so in others where the circulation is more vigorous (Bayly, 1965). The distribution of phytoplankton was found to be consistent with the physiography and estuarine circulation in the Port Hacking estuary in NSW (Scott, 1979).
It has been observed that some estuaries may exhibit a two layered circulation system with outflowing river flow over incoming tidal flows (Ranasinghe and Pattiaratchi, 1996; Stephens and Imberger, 1996). This pattern of circulation coupled with vertical migration by estuarine organisms may act to maintain a restricted horizontal distribution. For example, zooplankton were found to aggregate near the sediment on ebb-tides thus avoiding faster seaward currents in surface waters, probably promoting the retention of estuarine populations (Schlacher and Wooldridge, 1995). This phenomenon gave rise to marked differences in community structure between tidal phases. In tidal estuaries, temporal variations in plankton abundance at any given station are the combined outcome of pulses in active vertical migration and passive tidal dispersal (Schlacher and Wooldridge, 1995). Emergence of larval invertebrates was reported to be synchronized with lunar tides in an estuarine reach of the Brisbane River (Edwards, 1989). These examples emphasis the highly variable distributions of many estuarine organisms.
There are a range of other hydrodynamic processes that may also influence the distribution of estuarine organisms. For example, short-term variations in phytoplankton biomass were found to have been caused by both estuarine hydrological events resulting in the release of regenerated nutrients and to coastal hydrological events where slope water intrusions enriched the coastal waters and were introduced into the estuarine basins by tidal exchange (Scott, 1979). The nature of sampling programmes may influence the observed distribution of planktonic organisms in estuaries. For example, phytoplankton distribution was found to be almost uniform horizontally but the vertical distribution was rarely uniform in the Port Hacking estuary (Scott, 1978). This has implications for horizontal versus vertical trawl sampling and emphasises the need for appropriate sampling programmes to assess heterogeneous estuarine biota.
Variability in the distribution of estuarine biota is not restricted to the water column. On shorelines, the distribution of organisms is influenced by a range of physical factors. For example, it was reported that there was a general tendency for the vertical distributions of major groups of species to be shifted upwards with increasing exposure to wave action on NSW coastlines. The increased spray and splash at wave-exposed sites was found to increase the period of submersion during which the animals at the higher positions were able to feed (Underwood, 1981). There was however, an increase in the species diversity and densities of sessile organisms in the more sheltered portions of the coastline compared to more exposed sites (Underwood, 1981).
Extreme events may also lead to a greater level of heterogeneity of estuarine constituents and ultimately biota. Severe storms exhibited increased wave height, elevated fluid velocities, increased suspended solids, particulate organic matter, seston concentrations and increased sediment transport rates (Bock and Miller, 1995). Storms were also found to have influenced pioneer seagrass communities and succession in a southwestern Australian coastal lagoon (Kirkman, 1985). Storms and changes in sand movement were suggested to have maintained significant variability in an intertidal floral community in New South Wales (May, 1981). The nature of storm-induced disturbance and its relative impact on different biota was reported to have influenced recovery and the community structure of post-storm communities (May, 1981).
Severe storms are usually associated with rainfall and significantly increased river flow and estuarine biota may have evolved to take advantage of storm-induced flood events. For example, salinity in the Coorong, a narrow offline estuarine lagoon in South Australia, decreased after a period of above average flow in the Murray River. Ruppia flowered in response to the freshening of the lagoon. Some estuarine lagoonal macro-invertebrates were found to have colonised the normally hypersaline distal end of the lagoon (Geddes, 1987).
Severe flooding of the Calliope River in Queensland in 1974 (Saenger et al., 1980) and 1984 (Moverley et al., 1986) disrupted invertebrate communities, setting them back to immature pioneering communities. Recovery from these events showed cyclical recruitment and settlement with a linear increase in species and a logarithmic increase in abundance. At the extreme end of the impact of episodic events, evidence for earthquake-induced subsidence and tsunami causing major shifts from marshes and forests to mud flats and incipient tidal marshes was inferred from fossil diatom communities (Hemphill-Haley, 1995).
It can be concluded that the dissolved constituents in estuaries, particularly Australian estuaries, are highly variable under the influence of seasonal cycles and severe episodic events. Colonisation strategies and the adaptations and responses of estuarine biota to these variable patterns of constituents will be discussed in more detail in later Chapters.
This section briefly describes aspects of sediment input and distribution in estuaries. The relationship between anthropogenic sources of nutrients other contaminants, sediments and estuarine biota will be discussed more fully in Chapter 4.
The nature of the sediments plays an important role in the partitioning of substances between dissolved and particulate phases (McComb and Lukatelich, 1986). The particle size distribution of suspended sediment in streamflow is a function of catchment geology. Rivers in drainage basins dominated by granites and their weathering products, coarse sands, like the Harvey River in Western Australia, have suspended solids loads dominated by particles in excess of 60 µm. Rivers, like the Barwon River in eastern Australia situated in a basin comprised of deeply weathered clays, has a sediment load with a mean particle size of <2 µm (Thornton et al., 1995).
Removal of native vegetation and the establishment of urban and agricultural landuses has increased the volume of sediment reaching most estuaries and altered the particle size mix by changing the erodibility of the soil surface. Selective erosion preferentially moves finer particles into waterways and these finer particles are normally associated with proportionately higher nutrient and pollutant concentrations.
A number of estuarine characteristics govern the distribution of sediment particles in these highly dynamic environments (Sly et al., 1982) including:
The net effect of these processes is a distinctive sorting of the sediment load entering an estuary from landward with coarser particles being deposited in the nearshore area and finer particles being deposited in deeper waters offshore .It can thus be concluded that the distribution of sediment types throughout an estuary can be highly variable under the influence of parent geology and historical sediment supply from the catchment, inputs from nearshore environments and the flood tide delta, soil erosion and sediment inputs from cleared land and hydrodynamic processes within the estuary itself. In addition to these physical processes, there is a range of biological processes which may also have a profound influence on the pattern of sediment distribution. Biological action of deposit feeding benthic fauna and salinity effects of fine-particle aggregation and settling (Douglas et al., 1996), can act to modify the particle distribution in estuaries.
Water movement and the distribution of sediments in an estuary also have a controlling influence on the distribution of deposit-feeding and filter-feeding benthos. Species composition and richness was found to be strongly associated with sediment type (sand or mud) in Botany Bay (Jones and Candy, 1981). Hydrodynamic processes which alter the distribution of sediments may therefore influence the faunal communities associated with them. For example, increased water movement changed both the composition of sediments and macrobenthos from a community with an affinity for sandy mud to one with an affinity with stiff clays (Saenger et al., 1982). Sediment disturbance by hydrological processes was found to influence species assemblage structure of nematodes in the Tamar Estuary UK (Austen and Warwick, 1989). Sand movement was suggested as an important factor in the decline of seagrasses in Moreton Bay, Queenslandand (Kirkman, 1978) and an important factor in causing variations in intertidal floras in New South Wales (May, 1981).
Sediment resuspension and settling also play an important role in governing the distribution and abundance of benthic organisms, which in turn play a significant role in the nature of sediment resuspension. Sediment resuspension is primarily a physical process related to wave action, tidal currents, wind energy (Gabrielson and Lukatelich, 1985), bioturbation and the nature of macrophyte communities. Benthic deposit-feeders have the capacity to disturb fine sediments and this bioturbation may influence both the nature of the depositional environment (Schaffner et al., 1990) and enhance sediment erosion and resuspension.
The chemical composition of sediments plays a major role in the nature of benthic communities. Nitrogen and phosphorus compounds accumulate in estuarine sediments through coagulation and sedimentation of suspended particles delivered to the estuary in runoff, settling of organic detritus generated within the estuary and for dissolved P and NH4-N, through adsorption reactions in the water column followed by settling. Sediments normally have significantly higher concentrations of nutrients relative to the water column (Congdon and McComb, 1980). Sediment adsorption of N and P may account for much of the available nutrients, with adsorbed to interstitial ratios of 30:1 for NH4-N concentrations and 270:1 for PO4-P concentrations (Udy and Dennison, 1996). The interactions between chemical composition of sediments and their associated biota is discussed more fully in later chapters.
The distribution of biota in estuaries is influenced by a complex interplay of physical, chemical and biotic interactions operating at a variety of spatial and temporal scales. One of the few common characteristics of estuarine biota is that their distributions can be described as a spatial and temporal mosaic (Johnson, 1972; Neale and Bayly, 1974). Differences in the distribution of estuarine biota have been observed at spatial scales from metres to kilometres and for temporal scales from days to months (Morrisey et al., 1992a; Morrisey et al., 1992b).
Estuarine species have been classified into five groups, according to their salinity preferences (Day, 1981), including;
Species richness and diversity are normally greatest at the marine end of estuaries and decrease upstream ( Jones et al., 1986; John, 1988; Sheaves, 1998), although seasonal patterns may confound these relationships as physical processes and biotic interactions influence the growth and survival of organisms. Estuarine benthos in Tasmanian estuaries has been found to be widely distributed, with euryhaline marine and estuarine species dominating, and with marine species patchily intruding into estuaries at particular sites (Edgar et al., 1998).
This distribution of species and their salinity preference observed for Tasmanian estuaries, is probably typical throughout Australia where fresh species occur at the top of the estuary, euryhaline marine and estuarine species are found in the mid-estuary where salinities are most variable and a greater proportion of marine species occur at the mouth. This generalization may only apply to estuaries where patterns of salinity in estuaries are not subjected to significant seasonal cycles.
Communities of estuarine plankton, benthic microalgae and macrobenthos may be 'reset' after flood events in estuaries subjected to significant seasonal cycles in rainfall and runoff. Estuaries that have an annual fresh and saline phase may have much more complex patterns of faunal migration, establishment and distribution than typical estuaries described for the northern hemisphere and there may be significant horizontal displacement of both marine and fresh species at times of the year (John, 1987; John, 1988).
This chapter describes the response of estuarine biota to natural patterns, cycles and stressors and their distribution in undisturbed estuaries. The first sections describe some general aspects of the distribution of estuarine biota and the final sections describe issues specific to each trophic group. The influence of anthropogenic stressors on the distribution of estuarine biota is discussed in Chapter 4.
Physical and chemical factors influencing the distribution of estuarine biota have been described in the previous chapter, and in addition to factors described previously, there are a myriad of biotic factors which also influence the establishment, distribution and community structure of estuarine organisms. These may include; dispersal and recruitment, genetic structure, competition, predation, interactions with substratum characteristics (Furnas, 1995; Keough and Butler, 1995).
Soft bottom sediments have been described as displaying temporal and spatial mosaics of benthic infaunal communities of different maturity (Johnson, 1970; Poore and Rainer, 1979; Rainer, 1981). These same patterns have also been widely reported for other natural communities including fouling and shoreline assemblages (Saenger et al., 1979; Underwood, 1981) and seagrass meadows (Larkum and West, 1982).
It has been concluded that localized disturbances caused by runoff events, currents, wave action and other disturbances, physically disrupt communities and 'reset' them to a less mature state, where species diversity, size strata and stability are lower (Johnson, 1970). The phenomenon of disturbance leading to a spatial and temporal mosaic of mature and less mature communities has been observed in a range of ecosystems. These range from rainforests, where large mature trees fall and leave a patch for early colonists to exploit, to grazing and predation on benthic epifauna and colonial communities. Migration of blowouts has been suggested as a mechanism for maintaining seagrass meadows in a state of dynamic equilibrium (Larkum and West, 1982), where early colonizers move in to assist in the re-establishment of a more complex community (Cambridge and McComb, 1984).
Diversity at the local level (scales of 1-100 m) may be influenced by a range of biological and physico-chemical factors including the recent evolutionary history of the community (Harris, 1994), and even so called diverse mature communities may be subject to successful invasion of immigrant species (McCormick and Cairns Jr., 1992). The relationship between diversity and stability has been described from experimental evidence (Tillman and Downing, 1994), as non-linear with a threshold, after which no additional stability is gained from increasing diversity (Harris, 1994). There have been few published accounts of the nature of diversity-stability relationships and debate continues as to the general applicability of this rule (Hurlbert, 1971).
It has also been postulated that there may be a level of inbuilt redundancy in healthy diverse communities and that functional groups usually comprise multiple species with overlapping niche occupancy. There has been considerable debate amongst theoretical ecologists as to whether it is more important to retain functional groups, than it is to retain functional redundancy. Maintaining a large species pool with inbuilt redundancy, may increase the likelihood that an ecological service or function will be stable and continuing, despite natural or even anthropogenic environmental change (Cairns Jr., 1993b).
It has been concluded that in order to adequately define the nature of diversity-stability relationships, the community should be studied for at least one complete turnover of all individuals, including the longest-lived species (Connell & Sousa 1983 cited Underwood and Anderson, 1994). This would add significant overhead to investigations and probably in part, explains why there have been ambiguous reports in the literature. In practice, the assumptions of diversity and stability may be an oversimplification, particularly for Australian estuaries, because considerable variation in species composition has been observed in benthic communities in the absence of marked environmental perturbations (Poore and Rainer, 1979; Saenger et al., 1979).
The classical concept of succession consists of a progressive replacement of species where the early colonists disappear and are replaced by later colonists (Odum, 1969), with an ongoing increase toward a mature community, approaching a plateau in species richness and diversity. There are however, numerous estuarine examples that depart from this classical view of colonization and succession. It is instructive to recognize relationships between the nature of community structure, niche occupancy and the ability to accommodate stress, if the complexities of the successional process in Australian estuaries are to be understood.
Communities containing species that fulfill several functional roles in the ecosystem (e.g. shredders, grinders, predators, scavengers), are better able to integrate different forms of environmental stress, while communities with members occupying similar niches are more diagnostic of a particular stress (Cairns Jr. et al., 1993). The density and dominance of a particular group also appear to be strongly dependant on historical events (Posey, 1987). More recently, there have been a number of hypotheses advanced to describe in more detail, the distributions of organisms (McGuinness, 1984; Posey, 1987).
There has been an assumption in the literature that biological accommodation is most important in communities subject to lower levels of environmental stress and that physical control is most important in communities subject to higher levels of stress. Biological accommodation may include trophic group amensalism (Rhoads and Young, 1970), competition for space (Saenger et al., 1979) and adult-larval interactions. It has been further assumed that high diversity and stability result from biological accommodation, while lower diversity and instability result from physical control (various authors cited Rainer, 1981). In most benthic communities particularly in Australian estuaries, it is likely that community structure is determined by a combination of biological accommodation and physical control (Rainer, 1981).
It has been suggested from the results of monitoring intertidal organisms subjected to different levels of stress (sand movement and overturning), that none of the above hypotheses had primacy over others in all situations and in many situations no departure from random placement was found (McGuinness, 1984). Overall, physical disturbance, acting in a manner predicted by the intermediate disturbance hypothesis, seemed to be of greatest importance (McGuinness, 1984).
Sediments with greater habitat heterogeneity supported varying species richness compared to more uniform sediments (Jones et al., 1986). This observation is consistent with the stability-time hypothesis (Johnson, 1970), but this has been questioned in practice (Keough and Quinn, 1991). Succession in marine fouling assemblages was found not to have conformed to any one of the theoretical mechanisms of succession. Marine invertebrates and algae have shorter life spans than terrestrial plants from which the paradigm of succession is primarily derived (Greene and Schoener, 1982). Citing the results of investigations of fouling assemblages Underwood (1994), concluded that successional mechanisms only made sense in the light of the limiting resource in a system and on the traits of individual species (seasonal recruitment, growth, ability to resist invasion) which influence their ability to utilize the resource.
There are complex interactions between physical and biotic processes governing the dispersal and migration of estuarine organisms. Stochastic factors causing variations in recruitment may have considerable influence on the pattern of succession and community structure and composition (Davis and Van Blaricom, 1978; Underwood and Anderson, 1994). There are a number of models describing the reproduction and dispersal in estuarine biota. Many estuarine organisms can migrate vertically (Taw and Ritz, 1979; Schlacher and Wooldridge, 1995) and horizontally (Sheaves, 1993) over diurnal or seasonal patterns and be passively (Neira and Potter, 1994) carried into estuaries on flood tides. Motile organisms can alter their position in the estuary to suit changing physical, chemical, feeding or reproductive circumstances (Bayly, 1965; Rippingale and Kelly, 1995).
During periods of unfavorable conditions, larger organisms can migrate out of estuaries or to areas within estuaries where conditions are more favorable. For example, the blue manna crab (Portunus pelagicus) was found to have highly seasonal distributions in the Peel-Harvey estuary related to the strong seasonal salinity signals and breeding cycles (Potter et al., 1983). Non-motile organisms have also developed strategies to accommodate less favorable conditions, for example, bivalves such as Xenostrobis securis, close its valves during periods of unfavorable salinities (Wilson, 1969). Burrowing worms, and much of the Swan River estuarine biota, are euryhaline (Chalmer et al., 1976; Papas, 1994) and can tolerate a significant range in salinity.
The distribution of plankton in estuaries shows considerable heterogeneity of populations with variations in distribution according to developmental stage classes and sex. For example, the Copepod Isias uncipes from the Brisbane River estuary was restricted to the bottom where it was at high densities reducing rapidly in number moving away from the sediment (Bayly, 1965). The female component of estuarine copepods, Gladioferens, were found to undergo diurnal vertical migration in the Brisbane River estuary (Bayly, 1965).
The later sections of this chapter provide additional discussion of the migration and dispersal of organisms in particular trophic groups.
The establishment of biological communities in estuaries is influenced by patterns of immigration, establishment and competition following settlement (Poore and Rainer, 1979) and the interplay of seasonal cycles of reproduction (Underwood, 1981). Investigations by Underwood (1981) provide a specific example where significant seasonality was observed in the abundance of shoreline macroalgal species. He concluded that if recruitment of a given species of alga were continuous, and sufficient at all times of the year to replace individual plants that were lost, the species would show no seasonal pattern of distribution. If however, reproduction and recruitment were seasonal, there could be a seasonal change in the pattern of distribution without any seasonal change in the rate of mortality of individual plants (Underwood, 1981). In reality there may be seasonality in both the mortality of species and in reproduction and recruitment.
Immigration patterns for planktonic organisms mirror the prevailing hydrodynamic conditions. For phytoplankton, the presence of oceanic (Harris et al., 1987; Hallegraeff, 1995), or riverine (John, 1988), communities which are carried into estuaries, influences the community structure of the estuarine community. The 'jetting' of larval organisms along topographically stable fronts on flood tides has been found to influence the spatial patterns of recruitment in bays and estuaries (Kingsford and Suthers, 1996).
Once planktonic stages of benthic organisms have been carried into estuaries, their ultimate success depends on settlement and establishment. Substratum was found to have an influence on the patterns of establishment and change (Anderson and Underwood, 1994), but the effect of substratum on change became less pronounced over time. Organisms have been found to form distinct zones in situations where there is a gradient of physical conditions such as on sandy and rocky shorelines. In some situations however it has been found that significant spatial and temporal variations in intertidal communities confounded classification to distinct faunal zones (Haynes and Quinn, 1995).
There may also be considerable patchiness in the occupancy of primary substratum in situations where there are extreme events disrupting mature and colonizing communities (Underwood, 1981). Cyclical patterns of recruitment and settlement may be different for different species with settlement maxima occurring at different times for different species (Saenger et al., 1980). Following the severe disturbance in a riverine fouling assemblage, diversity increased linearly and abundance increased logarithmically up to a plateau phase after 29 months (Saenger et al., 1980).
Seasonal cycles may not show identical patterns from year to year particularly for situations subjected to regular extreme events. A period of drought and flooding showed that seasonal patterns were not repeated over years in the Hawkesbury estuary (Jones, 1987). It has been suggested that extreme events such as drought and flooding may 'reset' the biotic communities of estuaries in a spatial and temporal mosaic. The response of the community to extreme events may be different depending on the nature of the event. During drought, the number of species was found to be usually high and the community structure distinct (Jones, 1987).
The classical view is that colonization following severe disturbance consists of an initial rapid increase in opportunistic species followed by the slower establishment of equilibrium species which outcompete and reduce the numbers of opportunists (Odum, 1969). In practice, as mentioned earlier, the reverse may be true. Underwood (1994) found significant seasonal differences in settlement and the final composition of fouling assemblages. The progression of the assemblages to several possible stable endpoints was influenced by seasonal patterns of recruitment and the individual species ability to utilize and protect the limiting resource of space.
Early colonizers may grow rapidly and dominate the primary space to the exclusion of later colonizers (Underwood and Anderson, 1994). Conversely for periphyton communities, it has been shown that later colonists depend on early colonists for their survival (Brault and Bourget, 1985). In marine communities both inhibition and enhancement of later colonists has been shown (Dean and Hurd, 1980). An investigation of colonization dynamics in the Calliope River in Queensland reported significant periodicity in settlement of fouling organisms related to physical conditions (Saenger et al., 1979). Colonization consisted of a pioneer phase followed after 3-11 months by a climax phase. Mature communities on old fouling plates and on natural substrata in the area were a mosaic of pioneer and climax phases (Saenger et al., 1979).
An investigation of recovery following severe flooding of the Calliope River in Queensland showed a markedly different pattern of establishment with no clear pattern of opportunist colonization, only a slow increase in species diversity with superimposed seasonal signals until plateau densities were reached after 5 years (Moverley et al., 1986). There are no clear patterns of settlement and establishment for benthic epifaunal organisms in estuaries.The theory of early-successional species dropout does not seem to have relevance to these types of assemblages (Underwood and Anderson, 1994).
Selection following settlement and establishment has been found to be an important determinant of the composition of local populations of marine invertebrates (Ayre, 1990). Selection may include competition for food and space as the community matures and the effects of grazing and predation. Selection has been found to result in chaotic genetic patchiness in local populations of marine invertebrates with planktonic larval stages (Ayre, 1990). The diets of co-existing epifaunal species have been found in some instances to be broadly overlapping. For seagrass epifauna, diffuse exploitative competition was suggested as being a major structuring agent for the community (Edgar, 1990b). The nature of relationships between members of the same species has also been found to influence benthic organisms. The density of conspecific benthic organisms was also found to influence the ratio of sexual and asexual reproductionin (Stocker and Underwood, 1991) sessile marine invertebrates.
Food availability and predation have been suggested as influencing the community structure of benthic infaunal communities. In mesocosms that had been artificially defaunated, there were rapid increases in abundance of almost all species of epifauna (Edgar, 1990b) indicating that epifaunal communities may be food limited. Predation by fish was found to have no significant effect on keeping epifaunal communities below the carrying capacity, although caging artifacts may have confounded results (Martin-Smith, 1993) in this investigation. The nature of predation and the specific food preference of the predators have been found to influence the nature of benthic communities. Patchiness in a kelp forest was shown to be caused by pulsed herbivory by herbivorous fish compared to more continuous grazing by echinoderms (Andrew and Jones, 1990). A grazing gastropod had a significant negative impact on the colonization of one species of brown alga, but no effect on other species (Braley et al., 1991).
Stable carbon isotope determinations confirmed that two species of commercially important fish depended to varying degrees, upon seagrasses and epiphytes for their carbon source. Ratios of 13C/12C in the fish were more similar to those of seagrasses than for epiphytes, suggesting that direct herbivory of the seagrasses themselves was occurring (Nichols et al., 1985). Stomach contents of fish caught over vegetated areas in a southern Australian estuary showed that they ate only epifaunal invertebrates (Connolly, 1995). While in some situations, predators have been found to be highly selective in the food preferences, gut contents of fish species in Wilson Inlet showed that the relative proportion of ingested prey items in a particular habitat corresponded to the prey's relative abundance in the environment (Humphries and Potter, 1993).
Other interactions between planktonic organisms and the epibenthos have also been observed. For example, phytoplankton biomass was suggested as having influenced light levels reaching benthic macroalgae and ultimately its distribution in the eutrophic Peel-Harvey system (Lavery et al., 1991). Phytoplankton biomass in the Swan River estuary was only 3% of that observed in bioassay treatments with no added nutrients, suggesting that amongst other factors, grazing was suggested as having been responsible for the lower biomass (Thompson and Hosja, 1996).
The nature of predation has also been found to influence plankton abundance. For example, food supply, reproduction, growth and predation were found to have influenced the distribution of zooplankton in Westernport Bay, Victoria. Selective predation by planktivorous fish was found to have been controlling the distribution of Paracalanus indicus (Kimmerer and McKinnon, 1989). Species composition of zooplankton in the lower Swan River estuary was modified through selective grazing pressure (Hodgkin and Rippingale, 1971). Normal predator prey interactions can be modified by a number of physical factors including light availability and turbidity. High turbidity in Lake Alexandrina was suggested as having prevented size-selective fish from preferentially grazing larger zooplankton and invertebrate predation may have been insufficient to remove smaller zooplankton (Geddes, 1984b), thus maintaining a diverse community structure.
Mass mortalities are extreme examples of changes in the community structure of estuarine organisms. For example, the estuarine mussel Xenostrobis securis was found to undergo mass post-reproductive mortality in the Swan River estuary possibly caused by high salinities and temperatures. The probability of localized extinction of the community in the unstable estuarine environment was avoided by the presence of reproductive communities further upstream that did not suffer the same mass mortality (Wilson, 1969). There was a significant change in the species composition and abundance of encrusting algae following the widespread disappearance of sea urchins in Botany Bay, New South Wales. Following the loss of a significant element of the benthos, there were significant changes in benthic communities. Filamentous and foliose algae increased in abundance and crustose coralline algae decreased. Associated with this, there was a large but short-lived increase in invertebrate grazers (Andrew, 1991).
Niche diversity and habitat complexity of estuarine ecosystems profoundly influence the nature of biotic communities. More species and greater abundances have been found in vegetated habitats than in bare areas (Poore, 1982; Summerson and Peterson 1984; Connolly, 1994; Edgar et al., 1994; Gray et al., 1996). Similar relationships were found for fish with river banks having an abundance of tree roots and other snags compared to clear banks (Sheaves, 1996), suggesting that the relationship is not solely one of food supply and may include additional factors such as refuge, easier ambush, lower current speeds and lower turbidities.
Availability and diversity of food supplies were found to influence the distribution, abundance and species richness of benthic communities in seagrasses (Bell et al., 1988; Edgar, 1990a; Edgar, 1990b). The position of seagrass beds in estuaries was suggested as having a stronger influence on associated fish and decapod communities than the size and shape of the bed and leaf size (Bell et al., 1988), because of larval distributions and possible differences in predation.
The positive relationship between abundance, species richness and habitat complexity is not always consistent however. Density and biomass of fish were higher in Ruppia than for bare sand in Wilson Inlet, Western Australia, but the opposite was observed for species richness and species diversity (Humphries et al., 1992). The hypothesis that epifaunal abundance was related to habitat complexity was supported using results from macroalgal mimics with different levels of epiphytes and caging experiments to exclude predators (Martin-Smith, 1993)
Salinity has been found to have a major influence on the structure of species assemblages of benthos (Hodgkin and Lenanton, 1981; Jones et al., 1986; Austen, 1989; Austen and Warwick, 1989; Edgar et al., 1998; Papas, 1994) and diatom communities (John, 1988). Because of the influence of salinity on estuarine biota, ecological processes in large rivers and their receiving estuaries may be controlled by flow variability (Puckridge et al., 1998). For example, species richness and abundance of benthic macro-invertebrates showed significant intra-annual and inter-annual variability in all zones of the Hawkesbury estuary (Jones, 1987) and both were significantly related to river discharge. Zooplankton abundance in four Victorian estuaries was highly variable spatially and temporally and irregular streamflow in source streams was seen as strongly influencing the variability of the standing crop (Neale and Bayly, 1974).
Just as strong relationships between salinity changes and changes in community structure have been observed, situations where community composition has little obvious seasonality can be related to relatively constant salinities. For example, there was little evidence of seasonality in the species diversity or composition of benthos of the Yarra River (Poore and Kudenov, 1978b), Port Phillip Bay (Poore and Rainer, 1979), or Cabbage Tree basin, NSW, where temporal changes were relatively insignificant compared with those related to site differences (Rainer, 1981). A large and varied benthonic population of foraminifera was observed in Broken Bay, New South Wales, but mostly in section of the estuary not subject to large salinity changes (Albani, 1978).
Estuaries with predictable seasonal changes appear to have more predictable changes in biota (Jones, 1987), compared to estuaries with less predictable climates (Poore and Kudenov, 1978b; Poore, 1982; Jones, 1987; Rainer and Fitzhardinge, 1981). For example, temporal heterogeneity was found to be greater than spatial heterogeneity in the estuarine benthos of the Calliope River in Central Queensland during recovery after a major flood (Saenger et al., 1980). Conversely,
The impact of salinity on estuarine organisms may not always be consistent because of the range of strategies estuarine organisms have developed to accommodate variations in salinity. For example, zooplankton distribution in the Derwent estuary was found to be similar during periods of high and low freshwater flow (Taw and Ritz, 1978). No changes over time could be attributed to changes in salinity after rainfall for the benthos of a small NSW estuary, although heavy rainfall immediately prior to the study could have reduced some species at some shallow sites (Rainer, 1981) which may have distorted the results somewhat. This highlights the importance of having an understanding of the antecedent conditions when investigating estuarine biota
The effect of salinity on estuarine organisms may also vary according to physical factors such as depth. Salinity was observed to have had a greater influence on fish species and abundance in deeper waters than in shallow waters (Loneragan et al., 1987) in the Peel-Harvey estuary. It was suggested that this may have been caused by larger fish having lower tolerance to salinity changes than smaller fish in the shallows.
It may therefore be concluded that even though salinity changes can have a profound influence on the distribution, abundance and community composition of estuarine organisms, age-class differences in salinity tolerance and differential responses to the magnitude of salinity change and antecedent conditions, mean that inconsistent observations of the salinity response by organisms are likely.
Primary production in estuaries includes that of phytoplankton in the water column, attached periphyton and benthic communities of microalgae, macroalgae and seagrasses. The growth of estuarine plants has been found to be influenced by temperature, light, inorganic N and inorganic P (Gordon and McComb, 1989). Light is of critical importance in controlling the growth rates of pelagic and submerged plants and elevated nutrient levels may cause a marked increase in plant biomass (McComb and Lukatelich, 1986).
The response of estuarine plants to nutrients and light may be influenced by season and by the stage of growth of the plants. Amphibolis seedlings with limited root systems were found to have similar uptake rates for NO3-N, NH3-N and PO4-P to that of macroalgae from the same area, whereas adult seagrasses had generally lower rates of nutrient uptake when compared to macroalgae (Paling and McComb, 1994).
The standing crop of phytoplankton varies substantially in both space and time because of their patchy distribution. The patchiness of phytoplankton operates at a number of spatial scales and results from responses to physical and biological processes including (Stavn, 1971):
The most rapid growth or reproduction rates of phytoplankton are of the order of several hours, but whole populations generally require at least a day or more to double in size. Changes, which occur in a plankton community regularly every 24 hours, are referred to as 'diel' changes; daily and nightly occurrences are called 'diurnal' and 'nocturnal'. Included among diel changes are vertical migrations of motile phytoplankton, changes in photosynthetic potential and inshore changes in communities associated with tidal cycles.
Large-scale temporal variations are associated with seasonal cycles in the estuarine environment. Temperate waters generally display a greater seasonality in phytoplankton abundances than do tropical waters probably because of reduced light and temperatures in temperate waters during winter (Kennish, 1994). There may be inter-annual variability in peak phytoplankton abundances because of variations in factors such as wind strength, river flow, salinity and grazing pressure. Phytoplankton employ several osmotic mechanisms to ameliorate the effects of highly variable salinities in estuaries and estuarine communities have been found to be more euryhaline than coastal marine communities (Brand, 1984).
The annual production of phytoplankton averages about 50 g C m-2 yr-1 in the open ocean, from 50 to 250 g C m-2 yr-1 in coastal marine waters and from 10 to 500 g C m-2 yr-1 in estuarine waters (Kennish, 1991). The lower values in estuaries are associated with very turbid systems where light penetration is restricted. The depth to which plants can be mixed and at which the total photosynthesis for the water column is equal to the total respiration of primary producers is known as the 'critical depth'. The critical depth is controlled by mixing processes in estuaries and the level of turbidity in the water column.
Early ecological considerations of the uptake of nutrients by phytoplankton were firstly; that at low nutrient concentrations, the rate of nutrient uptake was found to be concentration dependant and secondly; that the total yield of phytoplankton was directly proportional to the initial concentration of limiting nutrient and independent of the growth rate of phytoplankton (Ketchum, 1939). More recently, it has been found however, that these simple relationships between nutrients in culture and phytoplankton, may not apply to open water systems. Assumptions of steady state at a variety of scales are essentially incorrect and nutrient limitation should be described in terms of fluxes, rate processes and growth and loss terms (Harris, 1986).
The primary determining factors for algal growth rate and final biomass are nutrients, light, temperature, micronutrients, sedimentation rate and grazing pressure from zooplankton. The apparent chlorophyll-a:TP ratio is a combination of a whole series of events in the food chain and the proportioning of N and P between a number of pools of varying size and turnover times. N and P limitation is not just a limitation of phytoplankton growth, but represents changes in the pools of N and P throughout the system (Harris, 1994).
A quote from Harris (1994 pp 36), best summarizes the roles of limiting factors in phytoplankton growth. "The accepted wisdom is that P limits rate processes and total biomass in freshwater and N does the same in marine waters, that light sets an upper limit on biomass when self-shading sets in, that temperature may limit rate processes in winter and that micro-nutrients may be ignored. …any of these factors may limit growth in isolation or in combinations".
In conclusion, the distribution, abundance and structure of phytoplankton communities may be highly variable in both space and time. Populations are subjected to the combined influences of physical/chemical boundary conditions including light, temperature and salinity gradients, advective processes, conspecific behavior within species and between species and selective and non-selective grazing pressures.
Benthic microalgae may make up a large proportion of the total biomass of estuarine microscopic plants (McComb and Lukatelich, 1986) and have been found to be up to 17% of the total production in a European estuary (de Jong and de Jonge, 1995). A number of factors have been shown to influence the establishment and productivity of periphyton and benthic microalgae. These have included; season, irradiance, concentrations of N, P and Si, tidal range and precipitation (Brotas and Catarino, 1995).
The surficial layer of sediments is a zone of intense microbial and geochemical activity and of considerable physical reworking. The vertical distribution of benthic microalgae is the net effect of the opposing actions of migration to the sediment surface by motile organisms and mixing which tends to produce a uniform distribution in the surface layer. The variability in vertical distribution may be confounded by considerable horizontal patchiness (MacIntyre et al., 1996). Distributions of viable benthic microalgae have been found to extend into the mixed layer of 15 mm (MacIntyre and Cullen, 1995) and more than 0.5 cm into surface sediments (de Jong and Colijn, 1994). MacIntyre (1995) reported that primary production was more or less equally distributed between the surficial millimetre of benthos and the overlying water and that vertical distributions of chlorophyll-a in sediments, varied by up to four times over scales of 1 to 10 mm (MacIntyre and Cullen, 1995). Chlorophyll-a concentrations in the 0-1 mm layer of sediment varied by up to 8 times on three successive days (MacIntyre and Cullen, 1995)
Periphyton colonization may take place as a number of distinct phases (Acs and Kiss, 1993), up to a plateau of equilibrium (Brault and Bourget, 1985). Numbers of individuals of certain species may collapse between colonization phases (Acs and Kiss, 1993), commensurate with decolonization rates (Brault and Bourget, 1985). The phases of colonisation would of course be accompanied by shifts in community structure. The losses of biomass were probably caused caused by water currents, changes in light reaching colonising species at the base of the assemblage, mechanical injury and grazing. Species composition of subtidal fouling assemblages was found to be influenced by grazing and time of commencement of experiments (Breitburg, 1985). Season clearly has a major influence on community composition of colonising organisms. For example there were marked temporal changes in the key taxa along a new Jetty in South Australia, but the spatial variability along the pier meant that it was difficult to draw statistical inferences about the temporal variability (Butler and Connolly, 1996).
The use of SEM micrographs has shown, that the surface of submerged substrata became coated by organic aggregates after the first week (Blinn and Korte, 1980). It was suggested that the organic matrix laid down early in the colonization process provides similar attachment surfaces for invasion thus reducing the initial micro-topographic differences displayed by different substrata and allowing for a more uniform colonization pattern in the second and subsequent weeks. Increasing microhabitat complexity (biotic heterogeneity) over time provides additional substratum for a greater range of species to colonize (Brault and Bourget, 1985).
It has also been found that there may be differences in the intensity of colonization of micro-phytobenthos between years (Brault and Bourget, 1985) and that initial colonization of fouling assemblages may be influenced by substratum type (Anderson and Underwood, 1994; Blinn and Korte, 1980). Colonization and decolonization rates were also found to be higher in summer than winter (Brault and Bourget, 1985), probably in response to higher grazing pressure and greater reproductive effort at higher temperatures. Periphyton community structure was also found to be influenced by current velocity in translocation experiments in Californian streams (Bergey et al., 1995) and in New Zealand (Biggs, 1995).
Nutrient availability has been found to have a marked influence on the establishment and growth of the micro-phytobenthos. Treatments combining C, N and P produced the greatest response in periphytic algae growing on nutrient-diffusing clay pots in an oligotrophic lake in Florida, USA (Havens, 1994). Periphytic communities were found to be limited by either N or P, or co-limited by both N and P in particular situations (Havens et al., 1996b). Diversity of the periphyton community was greatest for treatments producing intermediate biovolumes (biomass), with a marked reduction in diversity for treatments producing maximum biovolumes (Barnese and Schelske, 1994). This means that opportunistic species with higher growth rates dominated the community at higher nutrient levels to the exclusion of a number of slower growing species.
It can thus be concluded that colonisation and establishment of benthic and periphytic microalgae are the nett result of a complex set of dynamic interations between the presence of appropriate numbers of suitable colonists, the availability of suitable substratum, competition for space and resources, grazing pressure and erosive forces and antecedent successional history. Given the possibility that some or all of these factors may vary in space and time, it is not surprising that the distribution, abundance and community composition of benthic and periphytic microalgal communities are highly variable.
Zooplankton have an essential role in estuarine food chains as intermediate links between primary producing phytoplankton and secondary consumers. The grazing of zooplankton provides energy for higher-trophic-level organisms. Zooplankton have been implicated in the passage of particulate-bound heavy metals and synthetic organics from the water column to the sediments and to higher trophic levels (Schultz et al., 1995). Zooplankton communities are generally more abundant in estuaries than in neighboring nearshore areas (Bayly, 1965). Zooplankton abundance, productivity and fecundity are influenced by seasonal factors including temperature and salinity (Dias, 1994; Hoffmeyer, 1994; Turner, 1994), the nature and abundance of food organisms (Dam et al., 1994; Harris, 1994) and the nature of predation (Kimmerer and McKinnon, 1985).
Zooplankton have the ability to maintain populations in estuaries against the prevailing hydrodynamic schemes by vertical migration to areas of particular current direction. Light has been identified as a major factor in triggering diel vertical migration in these organisms. Illumination changes at sunrise and sunset act as environmental cues which trigger the migration process. Vertical migration patterns are complex with nocturnal, twilight and reverse migration patterns having been observed (Kennish, 1994). Migration patterns may also be influenced by life-cycle stage and reproductive status. For example, ovigerous females (in brood) appeared to participate less in diel vertical migration during periods of full moon than non-ovigerous females (Jerling and Wooldridge, 1992).
In addition to vertical and horizontal migration, zooplankton abundance may also vary seasonally, although the seasonal dynamics are not always predictable. For example, zooplankton abundance and community structure varied with season and salinity in the Swan River estuary (Hodgkin and Rippingale, 1971). Conversely, in semi-enclosed waters in another Western Australian estuary, zooplankton did not display marked seasonality in their distribution (Geddes, 1984a; Gaughan and Potter, 1995). Species composition of the zooplankton community changed with changes in salinity and temperature even though their distribution was unaltered (Gaughan and Potter, 1995). High nutrient loadings (hence phytoplankton biomass), high zooplankton densities, relative stability of the water mass and limited marine exchange were suggested as reasons for the lack of seasonality in their distribution.
Zooplankton growth rates vary significantly from less than a week for some protozoa to many months for some euphausiids. Populations of temperate copepods may have a single generation per year and normally grow from egg to adult in 2 to 3 months, depending on temperature and food supply. Metabolic rates in zooplankton are a function of temperature, which influences growth, sexual maturity, fecundity and longevity. Investigations of sex ratios in zooplankton communities in Brisbane River estuary found that females were usually more abundant than males in the less abundant species, but sex ratios were nearer 1.0 for common species (Bayly, 1965). Statistically significant variations in the coefficient of variation of egg numbers in estuarine copepods were found close to the station of maximum salinity tolerance (Bayly, 1965). The relationships between variability and total egg numbers were not clear however.
Initially, zooplankton were thought to be indiscriminate feeders or simply filter and ingest plankton communities. More recently it has been observed that zooplankton may be highly selective in their feeding preference. Zooplankton may influence the size classes of phytoplankton by preferentially grazing on smaller diatoms (Fahnenstiel et al., 1995), or by actively avoiding certain less palatable species (Paerl, 1988). The nature of relationships between primary producers and first-order secondary consumers is not simple however. A 38-day period of periphyton accrual, did not result in a treatment effect in patch enrichment studies on artificial substrata in New Zealand streams. There was however a two-fold increase in primary grazers with N and P enrichment, suggesting a tight coupling between first and second trophic levels (Biggs and Hickey, 1994).
The ability of zooplankton grazing to influence phytoplankton biomass has been used in successful top-down control of phytoplankton in European reservoirs through removal of predatory fish (Bernhardt and Clasen, 1985). There appears to be little scope for similar bio-manipulation of blue-green algal blooms in inland Australian waters, where native zooplankton are considerably smaller than their European counterparts (Boon et al., 1994) and removal of native predatory fish along the length of inland rivers, is both not possible or ethically sound (Gehrke and Harris, 1994).
In oligotrophic systems, much of the nutrient regeneration is influenced by pelagic zone zooplankton grazing and excretion (Harris, 1994), although the absolute amount of nutrient regeneration is influenced by the nutritional status of prey organisms (Vadstein et al., 1995). Again these relationships are not simple. For example in the meso-haline region of Chesapeake Bay, meso-zooplankton, suspension-feeders and fish as a whole, contributed relatively little on a seasonal or annual basis to the total amount of regenerated nitrogen (Baird et al., 1995).
In conclusion, estuarine zooplankton communities display significant vertical, horizontal and temporal variability. There are complex relationships between zooplankton migration, abundance, distribution and stage of development and reproductive cycles. Zooplankton community structure may also be highly modified through selectively grazing pressure by visual predators.
A benthic community may be defined as the assemblage of bottom dwelling species at a particular place and time (Johnson, 1972). Zoobenthic community structure may be influenced by the nature of the substrata, physical and chemical environmental processes, localized disturbances, recent evolutionary history of the community and recruitment and settlement dynamics. Benthic fauna are subdivided according to sieve mesh size into micro (<0.04 m), meio (0.04-0.1 mm), macro (0.5-2.0 mm) and megafaunal (>2.0 mm) components. The absolute abundance of micro and meio-fauna usually far exceeds that of the macrobenthos, but in terms of total biomass, the macrobenthos are normally in excess of the smaller benthic fauna.
The spatial distribution of benthic macrofauna is generally patchy at a number of spatial scales (Hutchings, 1990) and species composition and local distributions of benthic macrofauna have been related to various physical factors including currents and wave action, sediment characteristics salinity and depth, chemical factors such as dissolved oxygen concentrations and biological factors such as predation and competition.
The distribution of the macrobenthos in the sediment column has also been related to the successional development of the benthic community. In habitats subjected to frequent physical disturbance, pioneering infaunal species tend to dominate the fauna. Pioneering suspension feeders usually feed near the sediment surface or from the water column (Kennish, 1994). Habitats with less physical disturbance support more complex communities where deposit feeders become well established at a greater depth in the sediments. Spatial and temporal variations in species composition may be large in communities occupying harsh or heterogeneous environments.
Different levels of macro-invertebrate community stability have been observed in natural soft-bottom substrata when compared to hard substrata. Hard substrata not subjected to wave action generally support more mature communities with a greater relative diversity. Shallow-water benthic communities in soft substrata have been termed, low-grade communities, largely controlled by physical conditions (Johnson, 1972), or organic loadings (Horwitz and Blake, 1992), with a lesser influence from biological interactions (Rainer, 1981). The fauna of soft-bottom sediments are made up of a spatial mosaic of communities at different stages of maturity, constantly being 'reset' by localized, small-scale disturbance. These communities are characterized by low diversity, high abundance and smaller size strata (Johnson, 1970; Johnson, 1972; O'Connor and Lake, 1994).
Salinity has been identified as a significant factor in the distribution of benthic macrofauna (Chalmer et al., 1976; Austen and Warwick, 1989; Papas, 1994) although an investigation of benthic fauna in the Blackwood River, Western Australia found that salinity (< 6 g l-1) had little influence on community structure (Williams et al., 1991). This exception to a general rule may have been a special case where the Blackwood River has been subjected to considerable damage from agricultural clearing in the catchment and the fauna may have represented the halotolerant remnants of a previously more diverse fauna (Williams et al., 1991).
The distribution of benthic macrofauna is also a function of larval dispersal, settlement, establishment and survival. Many benthic faunal populations have planktonic larval stages to aid in the dispersal of the population. Planktonic larval macrobenthic organisms have very high mortality rates through predation and the vagaries of the physical hydrodynamic conditions. Some planktonic larvae can delay settlement and metamorphosis until favorable substrata are encountered. Bio-chemical triggers released by adult animals, particular substratum characteristics such as sediment grain size, presence of algal substrata and organic matter have all been implicated in triggering settlement and metamorphosis in larval benthic macrofauna (Kennish, 1994). It has been suggested that the patchiness in settlement of benthic suspension feeders may be caused in part by the adult-chemical-induced clumping during settlement, or because of patchiness in the distribution of suitable substrata. If one assumes that the distribution of suitable substratum is random, then this is in part consistent with the random placement hypothesis which postulates that the distribution of organisms is simply the result of random placement.
There have been five classes of benthic macrofaunal feeders identified; suspension feeders, deposit feeders, herbivores, carnivores-scavangers and parasites (Rhoads and Young, 1970). Most benthic macrofauna fall into the first two classes of suspension and deposit feeders inhabiting sandy and muddy bottom sediments respectively. Suspension feeders do not normally occur on soft muddy-bottom sediments as resuspension of sediments causes their filtering apparatus to become clogged with fine particles. Suspension feeders feed on plankton and detritus suspended in the water column.
Deposit feeders may be tube dwellers or free-living burrowers and feed within the sediments or at the sediment-water interface. They may be either selective or non-selective feeders. Selective feeders separate food items from sediments during ingestion whereas non-selective feeders ingest the bulk sediment, digest an organic component and excrete sediment and other non-digestible components. Deposit feeders primarily feed on bacteria, benthic microalgae, microfauna and meiofauna and detrital material in the sediments. This group of organisms is particularly important in estuaries as they continually rework the sediments and may alter the physical, chemical and biological characteristics of the estuary floor. Bioturbation by benthic macrofauna influences inter-particle adhesion, water content of sediments, bed roughness and geochemistry of interstitial waters. Both increased and decreased disturbance of sediments has been attributed to the actions of benthic macrofauna (Kennish, 1994; Rhoads and Young, 1970).
Diversity has been identified as a useful parameter in assessing the nature of benthic macrofaunal communities. According to the stability-time hypothesis (Sanders, 1968), the diversity of benthic communities is dependent on the stability of the environment as well as its recent history. For soft sediment benthos in Australian estuaries however, diversity and abundance are influencd by continual localised disturbances which have been found to 'reset' communities to a less mature patchwork. This makes the use measures of community structure difficult for ecological health assessment in the absence of considerable background information on the relationships between natural disturbance and community structure at a range of spatial and temporal scales.
In conclusion, macrobenthic community structure may be influenced by the nature of larval dispersal, settlement, establishment and survival, the nature of the substrata, physical and chemical environmental processes, localized disturbances, recent evolutionary history of the community and physical factors including currents and wave action, salinity and depth, chemical factors such as dissolved oxygen concentrations and redox discontinuities and biological factors such as predation and competition.
A number of different feedback mechanisms of biota on habitats have been recorded in the literature (see review by Nielsen and Jernakoff, 1996). These include:
Seagrasses and macroalgal turf have been found to alter current speeds and turbulence in shallow waters and acted to reduce resuspension (Gabrielson and Lukatelich, 1985) and enhance sedimentation (Fonesca et al., 1982). The effect of stabilization of sediment is not restricted to macrophytes and on a smaller scale micro-phytobenthos may play an important role in stabilizing sediment (MacIntyre et al., 1996). It has also been suggested that rather than being passive entities, strongly controlled by resource and physical stress limitations, some periphytic diatoms are able to ameliorate stress through the production of mucilage up to 10 mm in thickness (Biggs and Hickey, 1994). Marine fouling assemblages were shown to modify the surface topography of substratum to such a degree that they altered the patterns of current flow (Brault and Bourget, 1985).
Macrophytes and benthic microflora have been found to influence the flux of NH4+ from sediments and to thus impact on nitrification and denitrification rates. Seagrasses promote nitrification and denitrification rates through variable oxygenation of sediments and, because a significant proportion of their biomass is below ground, they can also influence water column and pore-water nutrient concentrations (Hillman et al., 1989). Dense accumulations of macroalgae were found to reduce dissolved concentrations in sediments, to promote the release of NH4+ and to impact adversely on the survival of benthic infauna (Gordon and McComb, 1989; Lavery and McComb, 1991).
Deposit feeders play an important role in nutrient regeneration and this may be a significant contribution to the estuarine nutrient balance. Both nitrification and denitrification have been found to increase because of the action of deposit feeders, bioturbating sediments (Nielsen and Jernakoff, 1996). The roots and rhizomes of seagrasses have been found to reduce the mobility of a variety of deposit feeders including; polychaetes, callianassid crustaceans, bivalve molluscs, echinoids and holothuroids (Brenchley, 1982), and consequently the action of deposit feeders in bioturbating sediments within seagrass meadows, would thus be restricted.
It has been observed that mobile deposit feeders may outcompete sedentary suspension feeders through disturbance, physical disruption of burrows and through an increase in resuspension of fine-grained sediment, which interferes with filtering mechanisms. Sedentary suspension feeders were able to exclude deposit feeders through tubes and colonies binding the sediment and causing physical obstructions.
It has been concluded that none of the popular hypotheses describing the nature of biological communities applies in all cases for benthic organisms in estuaries (McGuinness, 1984). Settlement and establishment are highly variable and established communities may form multiple stable states (Poore and Rainer, 1979; Underwood and Anderson, 1994) and a patchy mosiac of mature and less mature communities (Johnson, 1970; Rainer, 1981; Hutchings, 1990). It has also been stated (Underwood, 1994) that successional mechanisms for benthic organisms in heterogeneous environments only made sense in the light of the limiting resource in a system and on the traits of individual species. The nature of benthic communities in Australian estuaries may therefore be described as a combination of the random placement hypothesis at fine spatial scales during settlement and the intermediate disturbance hypothesis for established communities at slightly larger spatial scales.
More than 70 percent of the Australian population is clustered around coastal waterways (Arakel, 1995) and accordingly, there have been numerous published accounts of anthropogenic stressors causing adverse impacts in Australian estuaries. Much of Australia's runoff is discharged to estuaries and carries with it a range of pollutants. The following statistics describing the impact of pollutant inputs to estuaries and nearshore communities reveal the magnitude of the impacts. There has been a depletion of around 45,000 ha of seagrasses from Australian estuaries and nearshore waters caused by a range anthropogenic stresses (McComb and Lukatelich, 1986; McComb and Humphries, 1992; Walker and McComb, 1992) including increased discharges of nutrients, turbidity and thermal plumes from industrial outfalls. Sewerage systems discharge around 10,000 t TP yr-1 and around 100,000 t TN yr-1 and up to 85% of this enters receiving waterways from urban and rural areas.
Non-point source (NPS) discharges from urban and rural catchments carry with them sediments, nutrients, heavy metals, pesticides, oils and hydrocarbons and solid pollutants such as litter. Point sources such as industrial outfalls discharge to waterways and may contain any of the pollutants described above, together with polycyclic aromatic hydrocarbons (PAHs) and a wide range of other industrial chemicals and residues, depending on the nature of the industry and its waste water management practices. Industrial discharges may also carry a significant thermal load to receiving environments. Impacts of NPS and PS pollutants (Table 4.1) include; sedimentation and infilling of waterways, nutrient enrichment, organic loadings and altered benthic dissolved oxygen dynamics, increased contaminant cycling in estuarine biota and thermal effects (Hallegraeff, 1995; Keough and Butler, 1995).
| Table 4.1 Potential impacts and threats to coastal ecosystems in Australia. | |
|---|---|
| Impact | Affected |
| Reclamation and removal of vegetation | mangroves, saltmarsh, sand dunes |
| Port works and dredging | sandy, muddy and rocky subtidal areas |
| Industrial discharges | estuarine habitats generally, beaches |
| Alterations to drainage | saltmarshes, mangroves, estuaries |
| Exploitation | Habitat |
| Fisheries | estuaries, open sea, coral reefs, sandy subtidal |
| Bait, food, materials | rocky shores, estuaries |
| Oil, gas, ore mining | beaches, open sea |
| Sand mining | beaches and dunes |
| Recreation | Habitat |
| Fishing | rocky shores, estuaries, beaches |
| Off road vehicles | saltmarsh, dunes |
| Passive tourism | Coral reefs, beaches |
| Sea bathing | beaches estuaries |
| Pollution e.g. TBT, xenobiotics | estuaries |
| Public amenities and utilities | Habitat |
| Sewage | rocky shores, beaches, estuaries, sandy subtidal |
| Agricultural discharges | estuaries |
| Sea-level rise from greenhouse | all shallow water / intertidal / supratidal areas |
Litter washed in from creeks and drains discharging from urban areas, includes garbage, items in sewage, industrial wastes and packaging, plastic pellets and other discarded materials. The impact of these pollutants on estuaries and nearshore communities include a reduction of visual amenity and biota impacts through ingestion and entanglement. There have been few studies on litter inputs to waterways and the extent of impacts is poorly understood (Wace, 1995).
The state of Australian estuaries (738 in total) reflects the nature of inputs to them. In terms of water quality, 424 estuaries have been classified as excellent, 18 fair, 15 poor and 326 have no data (Saenger, 1995). There are real or potential threats to fisheries in 188 of the 783 estuaries and threats to conservation values in 178 (Saenger, 1995).
The following sections discuss the nature of anthropogenic stressors to Australian estuaries and the response of the biota to such stressors. It is important to note that there are potential complications when defining the nature of impacts on estuarine biota. For example, there are a number of estuarine species that have a wide range of tolerances to pollutants and that may be found in both undisturbed and in highly disturbed systems. Some species may be tolerant of one form of pollution, but very sensitive to another. Impacts on species may also vary over different developmental stages (Patrick and Palavage, 1994). These factors have the potential to confound any discussion of indicator species and any simple derivation of cause/effect relationships.
Anthropogenic disturbances may be either pulse or press perturbations or combinations of the two. Pulse phenomena are short-term, acute episodes of disturbance that are then removed. An example would be an oil spill, in which the incursion of oil is usually brief and removed. A press disturbance is one that is chronic and sustained, such as continuous discharges from a sewage outfall (Bender et al., 1984). The time scales and impacts associated with each form of disturbance may also depend on the life histories of the organisms being impacted. A pulse disturbance for a long-lived species may for example, be considered as a press disturbance for a shorter lived species.
A particular disturbance may also cause both pulse and press impacts on the environment. For example, dredging a shipping channel through a shallow estuarine entrance may cause a pulse disturbance from increased turbidity and suspended solids during its construction and press disturbance through increased current speeds and tidal ranges after its completion. In this context, some organisms may be adversely impacted by the pulse and other organisms positively or negatively impacted by the on-going press. As an example, where dredging altered the composition of sediments from sand to mud, species richness of macrobenthic infauna was found to have decreased, even though average species density per sample was unaltered by dredging (Jones and Candy, 1981).
There may be potential problems with the power of tests in this context because the variances of different variables being monitored may change relative to one another through time. Some of the issues accompanying the detection of impacts in heterogeneous systems are discussed further in Section 4.9.
Urban and rural developments have had a significant impact on the water balance, on sediment and nutrient inputs and on the nature of xenobiotic contaminant cycling in estuaries. Agriculture has been one of Australia's major industry sectors and contributes approximately 25 percent of total national exports in 1989-90. Agriculture also has the greatest impact on water resources of all sectors of the Australian economy (Alexandra and Eyre, 1993). It has been estimated that 77,000 tonnes of N, 11,000 tonnes of P and 15 million tonnes of sediment have been washed onto the Great Barrier Reef annually from Queensland's coastal catchments. This represents an increase from four to five times greater than the loads prior to agricultural development (Alexandra and Eyre, 1993). This phenomenon is common in other countries around the world and more than 80% of annual TP export was found to arise from diffuse sources in the Berg River catchment in South Africa (Bath and Marais, 1993). The following sections discuss the nature of nutrient losses from rural and urban catchments.
Clearing native vegetation and establishing rural and urban landuses has a significant impact on water balance and on the nature of catchment hydrographic responses. Development leads to an increase in impervious areas through construction of paved surfaces and because of compaction in rural areas. Rainfall does not infiltrate into the ground as readily and this leads to an increase in the volume and velocity of runoff. In addition, extensive networks of artificial drainage are constructed to remove stormwater from the land surface into receiving waterways as quickly as possible. Unfortunately, this has led to drainage networks that maximise local convenience and protection, without considering other important factors such as off-site damage from accelerated flow, water pollution, or even the loss of the water resource. Other problems associated with traditional stormwater conveyance through natural and constructed channels include increased channel erosion and downstream flooding, deposition of sediment and a resulting loss of property, wildlife habitat and natural vegetation (Livingston and McCarron, 1989).
The impact of clearing and rural and urban development leads to increased streamflow volumes and velocities, but impoundments may have the opposite effect. Impoundment of the river flowing into the Kromme River estuary in South Africa reduced average annual flows from 117 x 106 m3 to 2 x 106 m3 and altered estuarine salinity from a gradient (15 to 35), to a homogenous system of 35 and above. Biota changed from being plankton dominated to that of submerged benthic vegetation and macrobenthic invertebrates (Baird and Heymans, 1996). A study of artificial impoundments on streams in southeast Australia found that 49% were obstructed by some form of physical barrier (Harris, 1984). This caused major disruption to migratory fish and may also influence food web dynamics in estuaries. Hydroelectric impoundments were suggested to have reduced the flushing of the upper Derwent estuary (Davies and Kalish, 1994).
A period of drought reduced flow of the La Trobe River in 1983, which allowed investigations into its impact on estuarine processes. The decrease in flow saw a decrease in DO and an increase in dissolved solids. In this instance, there was however little alteration in the taxonomic richness of aquatic macroinvertebrate fauna (Chessman and Robinson, 1987). Further insight into the impacts of reduced flow to estuarine systems can be gained from investigations into the Gippsland lakes. Increased salinity would allow establishment of euryhaline species, but deoxygenation of bottom water and high benthic mortalities may occur if a salt wedge was maintained (Poore, 1982).
In their natural state, dissolved and particulate solids in streams are regulated substantially by the vegetation. Removal or damage to vegetative cover disrupts the linkages between streams and their catchments and leads to unregulated leakage of water, dissolved and particulate solids to receiving waterways (Lake and Marchant, 1990). In an undeveloped area, a natural stream normally adjusts so that its cross section and slope are in approximate equilibrium. Increased volumes and peak discharge rates of stormwater runoff produce drastic changes in natural stream channels. Accelerated channel erosion also creates downstream damage by mobilisation and deposition of eroded sediment. Lakes, reservoirs and estuaries fill, storm sewers and culverts become clogged causing flooding and leading to areas adjacent to streams becoming covered with mud and debris after floodwaters have receded.
Increased stream volumes and velocities associated with stormwater from urbanized areas produce more frequent floods. Areas that previously flooded once every five years may flood every year, or several times each year (Livingston and McCarron, 1989). These changes in stream velocity/frequency relationships may have implications for the survival of stream faunal communities and thus the processing and recycling of organic carbon and nutrients into riparian areas. The impact of sediment and pollutant loads on estuarine biota is discussed in more detail later in this chapter.
Clearing for agriculture combined with the application of artificial fertilizers has been responsible for nutrient enrichment of estuaries (McComb and Humphries, 1992; McComb and Lukatelich, 1986). The Murray-Darling Basin commission has estimated that in a wet year, approximately 70 percent of the P and 40 percent of the N reaching surface waters in the basin originate from agriculture (Alexandra and Eyre, 1993). Loading rates of nutrients to waterways may be up to 50 times those considered to be of cause for concern, using Vollenweider loading criteria (Geddes, 1984a).
Nutrients may be discharged to estuaries as dissolved or particulate forms and significant amounts of these inputs are potentially biologically available given appropriate hydrodynamic processes, settling, regeneration and uptake mechanisms. There is a lack of published accounts describing changes to nutrient delivery to estuaries probably because of a lack of reliable historical monitoring data. An example from the Richmond River estuary in NSW has described a 2-3 times increase in phosphate concentrations over the last 50 years, when comparing limited data collected during higher flows; and no change in nutrient concentrations for low flow periods (Eyre, 1997). It also appears, despite some past analytical uncertainty, that there has been a more than 6 fold increase in TP concentrations in the Swan and Peel-Harvey estuaries over the past 50 years (Deeley and Paling, 1996).
It may be difficult to ascribe in all cases, the sources of pollutants such as heavy metals and organic compounds, which may be delivered in point sources and as chronic low level loadings in non-point sources. Organic compounds in sediments and waters were investigated to determine the influence of sewage on Sydneys nearshore environment. In addition to the presence of coprostonal, a fecal sterol, petroleum products and combustion products including polycyclic aromatic hydrocarbons (PAHs) not normally associated with sewage discharges were detected (Nichols and Espey, 1991).
In the past there were frequent discharges of sewage to estuarine environments around Australia.Sewage treatment may include some or all of the following stages:
Because of a greater appreciation of the role of sewage in the nutrient enrichment of estuaries, town and cities have upgraded primary treatment plants and constructed secondary and tertiary treatment systems discharging to the marine environment or to land irrigation schemes. Sewage commonly discharged to the environment in the past consisted of a mixture of liquids and solids, containing domestic and industrial wastes, heavy metals, organic compounds, pesticides and pathogenic microorganisms. Pathogenic microorganisms in undisinfected sewage may include; bacteria, viruses, protozoa and helminths. These organisms may pose significant health threats to humans who either ingest contaminated shellfish or swim in contaminated waters. Potential diseases associated with poorly treated sewage inputs to waterways include; cholera, typhoid, poliomyelitis, dysentery and infectious hepatitis (Government of Western Australia, 1988).
There are currently fewer areas around Australia, where poorly treated sewage is discharged directly to streams or estuaries. It is somewhat difficult to quantify the cumulative impacts of past discharges of sewage to estuaries. In agricultural systems, past applications of nutrients to paddocks are considered to be important and fertilizer residual value functions are used to estimate the current availability of nutrients from past fertilizer applications. For estuaries, historical inputs of organic material and nutrients from sewage outfalls (that may have been recently upgraded or diverted), may leave a lasting legacy in receiving environments. For example, past sewage inputs may have played a role in increasing the overall productivity of an estuary and thus increased the probability of nuisance macrophyte and phytoplankton growth. Past sewage inputs may also have added to the available nutrient pool remaining in the estuary particularly for conservative elements such as phosphorus. Concentrations of the fecal sterol, coprostanol, have been found to provide a reliable indicator of relatively recent contamination of waterways by sewage (Leeming and Nichols, 1996), but it is not known whether these techniques would be useful in quantifying historical sewage inputs.
There is a very large array of chemical compounds, which may be discharged directly to estuaries or to streams discharging into them. Point source discharges are associated with significant exposure and impacts in close proximity to the point of discharge and lower level or chronic exposure some distance from the point of discharge. The dispersal of pollutants in point source discharges is governed by the nature of the pollutants in the discharge and the prevailing hydrodynamic processes. Particulate materials in point source discharges may settle in close proximity to the discharge point or be translocated some distance from the discharge point if settling times are lengthy or current speeds significant. Locally, the loading and partitioning behavior of sediment-bound contaminants is largely controlled by the nature and extent of interactions occurring at the sediment-water interface within individual depositional zones (Arakel, 1995).
Industrial discharges may also contain waste heat from industry. Industrial cooling waters have the potential to significantly change the temperature field locally or more widely if volumes are great and mixing and dilution processes restricted. There has been a number of investigations of thermal pollution in estuarine waters and their impacts are relatively well understood in some situations. A power station cooling water outfall in Spencer Gulf produced temperatures in the vicinity of the of plume of up to 28°C, which was equivalent to the highest field temperatures previously recorded for Posidonia (Ainslie et al., 1994).
Heavy metals may pose a threat to humans consuming estuarine biota, because of their persistence, their tendency to accumulate in the tissues of biota and because of the potential toxicological effects of some metals at higher body burdens. Although these elements are toxic to estuarine organisms above a certain threshold availability, many of them are essential to metabolism at lower concentration. Elements of concern as potential environmental contaminants even at relatively low concentrations include; Cd, Cr, Hg, Se and As. Other heavy metals of concern at higher concentrations include; Co, Cu, Fe, Mn, Mo, Va, Sr and Zn (Kennish, 1991). Concentrations and availability of trace metals in estuaries are controlled by many factors. In-estuarine processes such as advective transport, mixing, resuspension, and differential settling of metals adsorbed to sediments, also give rise to significant variations in the distribution of heavy metals throughout estuaries.
The ecotoxicology of heavy metals in the estuarine environment depends on their form in the environment as they may occur, bound to particles in the sediments, adsorbed to organic compounds in the water column, or dissolved as various ionic oxidation states. Many metals complex with organic compounds which influences their chemical speciation. The specific physico-chemical form of the element rather than its total concentration determines how it will behave in the environment. For example, in natural waters, chromium may exist in two interchangeable oxidation states, Cr3+ or Cr6+. The toxicity of Cr3+ is 10 times that of Cr6+ (Holdway, 1988). The global rate of mobilization of various heavy metals to the marine environment and their relative toxicities are summarised in Table 4.2. This table shows that Zn, Mn, Cu and Cr are the metals most commonly discharged to marine and estuarine waters while, Hg, Cd, Ag, Ni and Se are those having the greatest toxicities.
| Table 4.2 Mobilisation and toxicity of heavy metals in marine waters in decreasing order of toxicity (After Ketchum, 1980). | |||
|---|---|---|---|
| Element | Symbol | Mobilization109 g yr-1 | Toxicitya µg/l |
| Mercury | Hg | 4.1 | 0.1 |
| Cadmium | Cd | 3.0 | 0.2 |
| Silver | Ag | 11.1 | 1 |
| Nickel | Ni | 164 | 2 |
| Selenium | Se | 7.7 | 5 |
| Lead | Pb | 113.6 | 10 |
| Copper | Cu | 252.1 | 10 |
| Chromium | Cr | 201.5 | 10 |
| Arsenic | As | 72.7 | 10 |
| Zinc | Zn | 727 | 20 |
| Manganese | Mn | 257 | 20 |
Toxicitya Toxicity here is termed as that concentration above which deleterious effects may be observed for a range of estuarine biota.
Heavy metals originate from mining, industry and urban sources. Metal concentrations in estuarine waters may not reliably indicate the magnitude of inputs, as they are readily lost from the water column through biological uptake and adsorption onto suspended particles, which settle. It has been generally concluded that significant concentrations of dissolved metals in estuarine waters imply very recent inputs (Hosja et al., 1993; Batley, 1995). Monitoring errors for metals in waters are common, because of the difficulty of ensuring 'clean' analytical techniques at very low ambient concentrations.
Investigations into the historical pattern of metal discharge to estuaries through profile analysis have generally revealed that heavy metal concentrations in urban estuaries have been increasing (Deely, 1993). Aged sediments in Lindisfarne Bay, Tasmania showed around a 10 fold increase in Cu, Zn and Pb and an almost 100 fold increase in Cd over pristine conditions, following persistent discharges from an electrolytic zinc smelter (Wood et al., 1992). Recent reductions in metal concentrations in shallow sediments may have accompanied improvements in metal recovery from waste water (Wood et al., 1992).
Comparisons between two Tasmanian harbours, Bathurst Harbour (pristine) and Macquarie Harbour with a similar sized catchment, but degraded through inputs of mine tailings, are instructive as they give some insights into the behavior of metals discharged to the estuarine environment. Iron and zinc (dissolved and total reactive), were similar in the two estuaries. Very high loadings of particulate copper were observed in inputs to Macquarie Harbour, but these had sedimented before reaching the main harbour basin.
Nickel and cadmium in Macquarie Harbour were double the concentrations in Bathurst Harbour, and given low concentrations of these metals in inflows, it was not unexpected that levels were within those observed in the open ocean.
Manganese concentrations were up to an order of magnitude higher in Macquarie Harbour. Manganese appeared to be less influenced by adsorption processes as salinity increased and can thus be expected to have a higher proportion passing through estuarine systems (Mackey et al., 1996). Concentrations of copper were up to two orders of magnitude higher in Macquarie Harbour compared to Bathurst Harbour and exceeded environmental guidelines.
Concentrations of copper associated with organic matter were less than dissolved fractions in Macquarie Harbour indicating a greater potential for toxic effects. The reverse was observed for Bathurst Harbour where dissolved fractions were less than those associated with organic matter (Mackey et al., 1996).
Metals have considerable potential to be bioaccumulated and bio-magnified through the food chain. For example, Princess Royal Harbour was found to have elevated levels of lead in filter-feeding molluscs which exceeded all known health levels for foodstuffs, but these were not reflected in increased levels in the surface sediments inhabited by these molluscs (Talbot, 1983). The impacts of heavy metals on estuarine biota are discussed later in this chapter.
Organic compounds contained in fossil hydrocarbons, such as PAHs, together with pesticide compounds are not readily measured as soluble phases in waters. These compounds have very low water solubilities and inputs of these persistent pollutants are more readily measured in sediments or in benthic organisms. There has been a lack of consistent monitoring of these compounds in Australian estuaries and their impact on Australian estuarine biota is poorly understood (Connell, 1995).
Organic inputs from point sources may also include less toxic, persistent compounds such as wood fibre and sewage sludge. If large amounts of these materials are discharged into riverine reaches of estuaries for lengthy periods, problems can develop. Wood fibre and organic waste were found to have severely degraded the upper Derwent estuary (Leeming and Nichols, 1998), leading to low levels of DO and high concentrations of sulfide in saline bottom waters (Davies and Kalish, 1994).
The impacts of loadings of organic compounds to estuaries from sewage and other sources on estuarine biota are discussed later in this chapter.
There are a large number of environmental indicators that have been used in the past or that are potentially available to define aspects of the health of estuarine ecosystems. These were briefly discussed earlier and are summarised in Table 1.2. This section discusses the impacts of anthropogenic stressors on the distribution of estuarine plankton, benthic microalgae and macrobenthos, with a view to defining the suitability of these trophic groups as potential indicators of estuarine health (see Chapter 6). Examples from other trophic groups are also briefly discussed in some situations.
An accepted definition of estuarine health is the absence of ecosystem distress syndrome (Haskell et al., 1992). Characteristics of estuaries displaying ecosystem distress syndrome (Table 1.3) include: changes in community size spectra; changes in species richness and species composition; increased prevalence of opportunistic or pollution tolerant species and; increased incidence of disease or abnormalities (Rapport et al., 1985). While it may be possible to describe the characteristics of ecosystem distress syndrome after they have occurred, for prevention and management, it may also be necessary to identify the nature of environmental stress leading to the development of ecosystem distress syndrome and causal mechanisms (Glasby and Underwood, 1996). There is a need to identify "early warning" indicators, as opposed to those that simply describe past disturbances.
Assessments of rare species and the proportion of opportunistic species in a community have the potential to provide a degree of diagnostic precision, however using these measures to describe changes in the ecological health in estuaries may not be without difficulties. For example, it may be difficult to define the nature of environmental stress by using relative changes from k-strategists, to r-strategists because there may be no change in biomass (Rainer, 1981) and the size strata of opportunistic organisms (e.g. phytoplankton) may not always be smaller in undisturbed communities (Harris, 1994). In a North Sea benthic community, changes in the occurrence of rare species from mostly present, to mostly absent, were observed adjacent to oil production platforms, but it was only under severe pollution, that ratios of opportunistic species changed (Gray et al., 1990). This suggests that by the time rare species become absent from a community, considerable environmental damage may have already occurred.
It can be concluded that impacts on estuarine communities may be relatively easy to detect after large inputs of pollutants over long periods. For example, Port Phillip Bay has seen major increases in loadings of pollutants associated with sewage over many decades and these have led to local episodes of reduced DO, increased frequency, intensity, duration of phytoplankton blooms and toxic blooms and reductions in seagrass density (Chiffings et al., 1992). It appears that it may be difficult to describe the early onset of adverse changes to the ecological health of estuarine ecosystems. The following sections describe the impact of a range of pollutants on estuarine biota.
It is difficult to isolate the impacts of increased sediment and organic loadings to estuaries from those of nutrient enrichment because most discharges of sewage and stormwater runoff contain all three constituents. As discussed earlier, increased loadings of suspended solids to estuaries can originate from soil erosion and sediment transport from rural catchments and from poorly treated urban stormwater runoff. Processes leading to the remobilization of contaminated sediments in upstream reaches of a waterway may, through time, exert a significant influence on downstream water quality (Arakel, 1995). Increased flow velocities, alterations to estuarine bathymetry through the construction of navigation and channels and the loss of benthic communities, can also lead to increased resuspension of estuarine sediments, which may have a similar impact on estuarine biota to increased external supply of suspended solids.
Suspended solids loadings to estuaries are likely to impact on biota in a number of ways including; physical smothering, increased light attenuation and changes to dissolved oxygen fields if the suspended solids contain a significant proportion of refractory organic matter. The latter is discussed in the following section.
Physical smothering of organisms results when the loading of suspended solids settling onto benthic organisms, exceeds their clearance rates. For benthic plant communities this may reduce photosynthesis, and for suspension feeders, this may interfere with modes of feeding. For example, much of the particulate material in the water near a sewage outfall settled on the surface of the algal thalii reducing the amount of light incident upon the chloroplasts and thereby reducing the photosynthetic rate (Borowitzka, 1972). Smothering of barnacle spat by sediments was thought to have played a role in early mass mortality in a temperate estuary (Brault and Bourget, 1985). Large inputs of wood pulp waste from a paper mill on the Derwent lead to a loss of biomass and diversity of estuarine benthos, but some of this effect may have arisen from reduced dissolved oxygen supply (Horwitz and Blake, 1992).
Turbidity in estuaries may increase directly through discharge or resuspension of fine material in the water column, or through the indirect effects of increasing phytoplankton and epiphyte activity which reduce light penetration significantly (Lukatelich and McComb, 1986b; Chiffings et al., 1992; Walker and McComb, 1992). Increased turbidity in estuaries may also have the effect of reducing the compensation depth for phytoplankton and benthic plants. Reduced light penetration to estuaries, as well as impacts on plant photosynthesis, may impact on faunal communities (Edgar, 1990b). Loss of seagrass may reduce habitat diversity and thus the diversity and abundances of associated faunal communities (Walker and McComb, 1992). High turbidity levels may also influence relationships between size-selective visual predators and prey organisms (Geddes, 1984b) and thus influence community structure.
Eutrophication refers to the progressive enrichment of estuarine waters with inorganic nutrients. Estuarine systems most sensitive to eutrophication appear to be those that are characterized by poor circulation where oxygen depleted waters cannot be effectively oxygenated. Effected areas are often shallow coastal bays and estuaries with constricted entrances, that have low relative freshwater inputs and attenuated tidal ranges. The impacts of eutrophication include a shift from pelagic nutrient regeneration mechanisms to benthic nutrient regeneration mechanisms (Harris, 1994). Symptoms of eutrophication (Table 1.3) include changes in nutrient cycling, changes in primary productivity, simplification of food webs, reductions in species diversity, increased dominance of opportunists, changes in oxygen status of deeper waters (Rainer, 1981) and increased amplitudes of species populations (Havens, 1994; Birkett and Rapport, 1995; Nielsen and Jernakoff, 1996).
Four stages in the eutrophication process have been identified (Gray, 1992).
Eutrophication also includes increased occurrence of potentially harmful cyanophyte or dinoflagellate blooms, fish kills and an increase in the risk of shellfish poisoning (McComb and Humphries, 1992; Hosja and Deeley, 1993; Patrick and Palavage, 1994; Hallegraeff, 1995; Deeley and Paling, 1996).
Investigations in freshwater reservoirs and lakes (Williams and Wan, 1972; Thornton, 1987), have concluded that there may be a different eutrophication response in temperate and tropical waters. A concentration range of between 20-30 µg P l-1 phosphorus has often been used as the mesotrophic-eutrophic boundary value in temperate zone lakes and reservoirs. It has been suggested that this may be low when applied to tropical systems and a concentration range of 50-60 µg P l-1 has been suggested as being more realistic as the mesotrophic-eutrophic boundary for tropical lakes (Thornton, 1987). The reasons for the suggested differences in eutrophication response in tropical and temperate climates are not clear. Preferential zooplankton grazing is able to significantly modify plankton community structure (Hodgkin and Rippingale, 1971) and there may be tighter coupling between phytoplankton and zooplankton grazers in tropical systems. Phytoplankton and zooplankton abundances are generally more consistent throughout the year in tropical systems compared to significant seasonal differences and winter minima in phytoplankton abundance in temperate systems (Parsons and Takahashi, 1973). It is not clear whether there is in fact any real difference in the eutrophication response in temperate and tropical estuaries, because of a lack of comparative data (Harris, 1994) and greater tidal ranges and flushing in tropical estuaries compared to temperate estuaries (Figure 1.2), tend to confound these types of comparisons.
There have been numerous published accounts of the impacts of eutrophication in Australian estuaries. Selected examples for plant community response include: a change from Hormosira to opportunistic Ulva in sub-tidal macroalgal communities following discharges of secondary treated sewage (Bellgrove et al., 1997) and a reduced number of plant species close to outfall compared to more distant sites (Brown et al., 1990). Artificial substrata accumulated greater biomass of periphyton adjacent to a sewage sludge outfall where seagrasses had been lost, than in areas remote from the outfall where seagrasses were intact. Increased epiphytic shading of seagrasses adjacent to the sludge outfall was suggested as the cause of seagrass loss (Neverauskas, 1987a; Neverauskas, 1987b).
Because the impacts of organic enrichment are the net effect of loading and flushing by hydrodynamic processes, it is not surprising that there are published accounts that have found little impact from sewage inputs in certain locations. For example, the natural variability in intertidal communities was found to be greater than that induced by the presence of low volume well-treated sewage (Smith, 1994), except in the immediate area of the outfall (May, 1981).
As discussed previously, the Australian environment displays a greater level of climatic heterogeneity than other areas in the world and estuarine processes are highly dynamic. It follows that defining the onset of symptoms of eutrophication in Australian estuaries, may be accompanied by a greater level of uncertainty than elsewhere.
Defining the onset and progression of eutrophication may require an assessment of relative change in the spatial and temporal occurrence of the symptoms of eutrophication in the system. It is possible that pristine systems may have had episodic events with very low recurrence intervals that resemble the symptoms of eutrophication. For example, phytoplankton or macroalgal blooms may have followed a severe bushfire and runoff event on a pristine catchment, which would have delivered a larger-than-normal nutrient load to a waterway (Cullen and O'laughlin, 1982). The pristine system would probably have recovered quickly from this type of short-term event. A spatial example of the symptoms of eutrophication in pristine systems would come from small areas, such as blind channels and pockets with restricted circulation, where organic detritus may have accumulated under wind action and localised periods of anoxia and algal proliferation may have occurred.
It can thus be concluded that eutrophication as it is now defined is not simply the presence of symptoms of eutrophication spatially or temporally, but rather a relative increase in the frequency, intensity, duration and extent of the symptoms of eutrophication in Australian estuaries.
It is also possible that the symptoms of eutrophication may be triggered by episodic events in systems experiencing moderate levels of nutrient enrichment. Primary production and chlorophyll-a standing crop were found to have increased about 40 fold during a period of non-upwelling and unseasonally low river flows in the Paramatta estuary. It was during these unusual circumstances that the impacts of cultural nutrient enrichment became most obvious (Revelante and Gilmartin, 1978). Additionally, blooms of phytoplankton followed the loss of zooplankton grazers in freshwater lakes (Havens and Hanazato, 1993).
As described above, nutrient enrichment generally leads to an increase in plant biomass in estuaries (Geddes, 1984a; McComb and Lukatelich, 1986). Increased plant biomass may include: an increase in phytoplankton (mostly diatoms John, 1987), although in severe cases, potentially harmful dinoflagellates and cyanophytes may occur (McComb and Humphries, 1992; Hosja and Deeley, 1993; Harris, 1994); increased growth of submerged macroalgae (Lukatelich et al., 1987; EPA, 1990a; Lavery et al., 1991); and increased production of opportunistic seagrass species (Connell, 1975; Lukatelich et al., 1987). Increased growth of opportunistic plant species in estuaries may cause the loss of seagrasses (Walker and McComb, 1992; Brodie, 1995), through smothering by macroalgal blankets (Gordon and McComb, 1989; EPA, 1990a; Lavery et al., 1991), or through reduced light levels caused by increased epiphyte biomass (Cambridge and McComb, 1984; EPA, 1990a; McComb and Humphries, 1992; Neverauskas, 1987a; Neverauskas, 1987b). There has been considerable investigation into the level of nutrient supply in estuarine waters that trigger responses in estuarine primary producers (Table 4.3).
| Table 4.3 Typical concentrations of nutrients reported in the literature for severe phytoplankton blooms. | ||||
|---|---|---|---|---|
| Site | DIN | PO4-P | Chlorophyll-a | Cell density |
| Peel-Harvey estuary | 600 (3000) | 20 (200) | 20 (60) | >106 |
| Lake Alexandrina | >200 | 50 | 40 | |
| Numbers represent median condition, numbers in brackets represent peak values. | ||||
The presence of opportunistic species and phytoplankton blooms however may not always be associated with eutrophic conditions. Blue-green algal blooms have been traced back to the middle ages and were thought to be a natural component of aquatic systems. These phenomena may however, reflect the impacts of medieval agriculture, or long term nutrient enrichment from natural sources (Anderson, 1995). Nodularia blooms were recorded from Lake Alexandrina from the mid 1800s (Francis, 1878 cited Codd et al., 1994) which were clearly before any significant anthropogenic influences.
Estuarine organisms have evolved mechanisms to take advantage of increased nutrient or organic matter supply. For example, the dominance in summer blooms in the Swan River estuary by motile species, particularly marine dinoflagellates (John, 1987), may reflect a competitive advantage of these species. Dinoflagellates migrate vertically between nutrient-rich bottom waters and near surface waters with higher average irradiance (Thompson and Hosja, 1996). This is compared to less motile species, such as diatoms, which are subjected to advective forces which may move them away from the photic zone for extended periods.
It has been observed that photosynthesis in benthic macrophytes may be reduced as turbidity and light attenuation increases (Neverauskas, 1987a; Neverauskas, 1987b; McComb and Humphries, 1992). This means that increasing phytoplankton biomass would tend to increase light attenuation and this would have an adverse impact on the productivity of benthic macrophyte communities. It may be speculated that benthic microalgae should also be disadvantaged in the presence of high phytoplankton densities and hence high light attenuation. The reverse has been observed however and benthic microalgae may have evolved mechanisms to survive occasional periods of low light levels. It has been found that there are only poor relationships between the biomass of benthic microalgae in the field and ambient light levels (Shaffer and Sullivan, 1988). It may be possible that some species of benthic microalgae become heterotrophic in situations where light is limiting.
There have been few published Australian investigations into the relationships between zooplankton and benthic macrofaunal distribution and organic and nutrient enrichment. Positive relationships were observed between TN and zooplankton biomass in the nutrient-enriched freshwater Lake Okeechobee, in Florida, USA. This was thought to have been caused by N limitation of primary production or food sources for the zooplankton community rather than any direct nutrient effect (Crisman et al., 1995). For benthic communities, there have been several investigations into faunal changes adjacent to sewage outfalls (Poore and Kudenov, 1978a), or overseas around sewage sludge dumping grounds (Pearson, 1984, cited Clarke and Warwick, 1994).
Sediments adjacent to the Werribee wastewater treatment plant outfall were found to be devoid of benthic fauna, with an increase in abundance and richness with increasing distance away from the source (Axelrad et al., 1979). Opportunistic deposit-feeding polychaetes and amphipods dominated the fauna in proximity to the outfall (Poore and Kudenov, 1978a). In the North Sea, Similar patterns of benthic macrofaunal distribution were found around sewage sludge dumping grounds with the lowest faunal abundance and species richness coincident with the dumpsite. Both abundance and diversity increased to a maximum moving away from the dump site, then decreasing slightly some distance from the dump site. The zone of maximum richness and abundance contained, in addition to species typical of undisturbed communities of the area, opportunistic species found adjacent to the dumpsite (Pearson, 1984, cited Clarke and Warwick, 1994). The observation of highest species richness occurring at the boundary between communities in undisturbed and highly disturbed situations is consistent with the intermediate disturbance hypothesis (Connell, 1978; McGuinness, 1984).
While investigations have found clear relationships between benthic faunal distribution and organic loadings, the impacts of increased nutrient concentrations in the water column are less consistent. Increased nutrient loads have been associated with reductions in biomass and species richness and changes in community composition and size-structure (Gray et al., 1990; Gray and Pearson, 1982), but clear relationships between dissolved constituents in the water column and benthos have not been demonstrated (Poore and Rainer, 1974; Poore and Rainer, 1979). Evidence has been presented in support of positive relationships between macrofaunal production and TN, DIN and NO3 and a negative relationship for NH4 (Nielsen and Jernakoff, 1996). Causal mechanisms for a rapid decline in a population of Katelysia spp. observed in Princess Royal Harbour, Western Australia which accompanied with nutrient enrichment, seagrass dieoff and macroalgal blooms were not established (Peterson et al., 1994), but the dieoff may be related to changes in NH4-N and DO concentrations which often accompany severe nutrient enrichment.
The effect of low dissolved oxygen causing adverse impacts on benthic macrofauna has been well documented in the literature. Increased nutrient loads have been associated with increased periods of hypoxia/anoxia and benthic mortalities (Gray, 1992), particularly where saline stratification occurs (Poore, 1982). Reduced dissolved oxygen in bottom waters may lead to localised extinction of benthic communities, reduced growth rates and changes in benthic community structure (Forbes and Lopez, 1990; Nielsen and Jernakoff, 1996). While there are clear negative relationships between faunal abundance and dissolved oxygen concentration in bottom waters, different macrofaunal groups show different tolerances to anoxia/hypoxia. Overall, deposit-feeding polychaetes were found to be least affected by low dissolved oxygen concentrations (Nielsen and Jernakoff, 1996).
The impact of low dissolved oxygen concentrations on other fauna has also been investigated. Fish and other highly mobile species are able to avoid anoxic waters and may in fact take advantage of stressed benthic macrofauna. Anecdotal evidence from fishermen in the Swan River estuary have found the best catches of Black Bream and Mulloway occur at the leading edge of the saline wedge (L Harboard pers. Comm.). This has been associated with low dissolved oxygen concentrations (Stephens and Imberger, 1996) and benthic macrofauna may emerge from burrows and tubes during periods of reduced dissolved oxygen concentration (Nilsson and Rosenberg, 1994).
System-wide investigations into relationships between nutrient increases and catches of commercially important fish species over many years may provide a broad insight into changes in the productivity of estuaries. It has been observed that TP concentrations the Peel-Harvey and Swan estuaries have increased over the past 50 years (Deeley and Paling, 1996) and there has also been increased catches of fish in recent years (Lenanton et al., 1984). The abundance of fish in the Peel-Harvey system increased by 1.8 times along with an increase in macroalgal biomass. Fish growth rates were similar to those of the Swan River estuary where was a 1.2 times increase in fish abundance and there were no large macroalgal beds (Lenanton et al., 1984). The greater increase of fish abundance in the Peel-Harvey estuary may have been related to an increase in invertebrates associated with the macroalgal beds. Increases in macroalgae in estuaries arising from nutrient enrichment may also cause adverse impacts for invertebrate communities. For example, drifting algal mats were found to have significantly reduced the settlement and establishment of benthic macrofauna (Olafsson, 1988).
It can thus be concluded that nutrient enrichment may have varying impacts on benthic macrofauna. Mild nutrient enrichment may lead to an increase in the abundance and productivity of benthic macrofauna through increased food supply, whereas severe nutrient enrichment may lead to increased concentrations of NH4 and decreased dissolved concentration concentrations in bottom waters, which would impact adversely on benthic macrofauna.
The following section describes the impact of thermal pollution, acid-sulphate runoff and toxicants on estuarine biota.
There have been few investigations of the effect of thermal plumes on estuarine biota in Australia. Electricity generation from fossil-fueled stations is generally around 40% efficient, which means that around 60% of the heat energy generated is lost to the environment, much of this through cooling waters. Thermal impacts are likely to be most acute where the volume of cooling water is high relative to the dissipative capacity of the receiving waterway. Shallow, semi-enclosed, poorly-mixed estuarine waters are most vulnerable to thermal inputs. Though it is possible that thermal discharges could selectively eliminate large components of a healthy aquatic ecosystem, most of the impacts appear to be less pronounced except in the mixing zone of the outfall. Other water properties such as; density, viscosity, surface tension and DO solubility also decrease with increasing temperature and these may have implications for stratification, mixing and hydrodynamics in estuaries. In the vicinity of outfalls, events such as heat shock and cold shock resulting from tidally-induced changes in current direction and flow, may lead to stress and increased mortality of biota. Thermal pollution may in certain circumstances, also induce dissolved oxygen stress, because warmer waters contain hold less dissolved oxygen and respiratory requirements for oxygen may be higher in warmer conditions.
Thermal loading may reduce primary production, change community respiration, species composition, nutrient dynamics and secondary production in estuaries (Hall et al., 1978). For example, Posidonia sinuosa had significantly reduced productivity, standing biomass, blade length and blade growth rate adjacent to a thermal plume. Growth response of Posidonia adjacent to the thermal plume was typical of its behavior in marginal natural environments. Posidonia displayed reduced productivity, standing biomass, blade length and blade growth in the naturally warm waters of Port Paterson, where summer temperatures were marginally higher than those of the thermal plume and where intermittent peak temperatures exceeded 30°C (Ainslie et al., 1994).
Thermal discharges may also influence community structure of benthic microalgae (Chessman, 1985) and in phytoplankton communities. For example, chlorophytes and cyanophytes may have an advantage over bacillariophytes (diatoms) in warmer waters and tropical waters may be more susceptible to thermal pollution than temperate waters (Hall et al., 1978). Zooplankton metabolism depends on temperature, which strongly effects overall physiology and ecology. Higher temperatures in the thermal plumes may result in sub-lethal impacts such as diminished growth and lower biomass in zooplankton (Kennish, 1991). Species richness of benthic macrofauna may be reduced and productivity may be either reduced or increased adjacent to thermal plumes, depending on ambient conditions, the nature of the benthos and on the severity of the thermal pollution (Saenger et al., 1982; Kennish, 1991).
Clearing of land with high concentrations of pyrite together with drainage and flood mitigation works have caused acid-sulphate drainage in eastern Australia and very low pH of estuarine waters following runoff events (Lin et al., 1995; Sammut et al., 1996). Strong tidal action was found to have favoured the enrichment of pyrite in the sediments of the estuarine plain. It was suggested that the acidified pyritic layer could act as a storage sink of acid sulphate materials which were able to be transferred upwards into non-pyritic overlying sediments through capillary action, thus causing acidification of overlying sediments (Lin et al., 1995).
Impacts in estuarine waters include very low pH values for many weeks following runoff events, iron flocs coating estuarine biota and very low pH values (~3.0). Acid drainage was found to have impacted on the entire 90 km length of riverine reaches and monomeric aluminium was observed to be over 300 times greater than ANZECC guidelines in the Richmond estuary following an acid-sulphate runoff event (Sammut et al., 1996). Ecological impacts of acid-sulphate drainage or acidification on biota have included (in increasing severity); a shift to smaller tolerant opportunistic species, increased prevalence of disease and mass mortality of worms, crustaceans, shellfish, fish and aquatic macrophytes (Havens and Hanazato, 1993; Sammut et al., 1996).
Heavy metals, pesticides, halogenated hydrocarbons and petroleum hydrocarbons in the world's marine and estuarine waters have long been recognized as some of the most potentially deleterious contaminants to biota and to human consumers of seafood (Martin and Richardson, 1991). Cumulative impacts of persistent contaminants have had dramatic effects on water quality and thus on ecosystem structure and functioning (Arakel, 1995). There have been a range of impacts of xenobiotics reported in the literature including increased frequency of embryonic malformations and chromosomal aberrations in developing fish eggs collected adjacent to point and non-point source discharges (Klumpp and von Westerhagen, 1995). Chronic low-level inputs of toxicants may alter the evolution of phytoplankton and zooplankton communities by eliminating sensitive species. Phytoplankton may also play an important role in the cycling of bio-accumulative materials to higher trophic groups and the efficiency with which phytoplankton can act as agents of bioaccumulation and trophic transfer, may govern the ecosystem-level impacts of toxic materials (Cloern, 1996).
The nature of heavy metal discharges and their behavior in Australian estuaries has been discussed in Section 4.3.2. Concern has been expressed over the level of analytical precision required to determine concentrations of metals in estuarine waters, sediments and biota. It has been concluded (Batley, 1995), that most of the published accounts of metal behavior in the Australian estuarine and marine environment must be questioned because of the lack of precautions taken to avoid contamination during sampling and because there are few laboratories with the demonstrated capacity to undertake ultratrace metal determinations.
It has been concluded, that it may be better to use metal concentrations in sediments or biota as indicators of pollutant loading, rather than concentrations in waters because of the requirement for ultratrace laboratory techniques. Other complications in determining the ecotoxicology and impact of metals in the estuarine environment arise because of; the persistence of metals in the environment, the potential for bio-accumulation, the potential for bio-magnification of some metals, the large range of metal-organic compounds that exist in the environment, the variable and unknown toxicities of most of the different metallic forms and a lack of information on synergistic effects of metal cocktails.
While bio-accumulation bio-magnification implies that it may be easy to detect concentrations of heavy metals in biota it may be difficult to determine the impacts of particular body burdens of heavy metals on estuarine biota. Human health limits for heavy metals in edible estuarine biota have been established, but there is little available information on the impacts of acute and chronic exposure to heavy metals on the biota themselves, particularly for Australian estuarine biota. Some examples of relationships between heavy metals and estuarine biota are presented below to illustrate their complexity.
Active uptake of essential trace elements including; Cu, Co, Cr, Fe, Mo, Ni, V and Zn, by phytoplankton may be significant especially during blooms. Phytoplankton utilize these elements in metal-activated enzyme systems. Zooplankton facilitate the removal of heavy metals from the water column through grazing and by consolidating them in fecal pellets. Fecal pellets, together with crustacean molts and dead animals and plants, may be responsible for more than 90% of the settling of these elements from the water column (Bryan, 1976).
Suspension-feeding benthos may trap and accumulate metals through filtering organic material, containing adsorbed heavy metals. Bioturbation of surface sediments by benthic fauna plays an important role in the redistribution of heavy metals in estuarine sediments. The impact of heavy metals on estuarine biota is highly variable, depending on the concentration, form and toxicity of the metals, the type and duration of the exposure and the life history and stage of growth of the organisms.
For example, chromium concentrations of 20 µg l-1 have been found to reduce the photosynthetic activity of algae (Hart, 1982) and concentrations as low as 10 µg l-1 can produce sub-lethal effects on the crustacean Daphnia magna. The duration of exposure to heavy metals has a significant impact on toxicity. For example, for Capitella capitata, the 4 day LC50 of 5,000 µg Cr l-1 fell to less than 500 µg Cr l-1 for a 28 day exposure (Reish, 1976, cited Mance, 1987).
The response of soft-sediment fauna to metal accumulations is not consistent. For example, artificially enhanced copper concentrations in sediments produced inconsistent results across taxa, with some taxa decreasing in numbers relative to controls and others remained constant, while the controls decreased in numbers (Morrisey et al., 1996). Crustaceans, bivalve molluscs and larval polychaetes, have been found to be more susceptible to Zn than adult polychaetes, gastropod molluscs and fish (Mance, 1987). Marine fish have been found to have a relatively high tolerance to Zn in waters when compared to freshwater fish and juvenile marine fish are only slightly more susceptible than adults (Mance, 1987).
The toxicity of a given metal once ingested, depends on the bio-chemistry and metabolic pathways of the organism concerned. Heavy metals may be maintained in a metabolically available form within the organism, or if accumulated at higher levels, cellular detoxification through complexing with proteins or fats may occur (Kennish, 1991). Chronic effects of high levels of stored heavy metals in organisms include alterations to growth patterns and reproductive processes. There is also the potential for populations of organisms constantly exposed to high levels of heavy metals to develop a measure of tolerance through biological accommodation possibly through genetic selection of resistant traits in the population. In these situations, body burdens that may produce sub-lethal effects in sensitive populations may not produce any observable effect in biologically accommodated populations.
The main source of metals in estuarine waters has been found to be from the mobilization of surface sediments (Williamson et al., 1996). The nature of on-going exposure to heavy metals in estuaries is influenced by sedimentation and resuspension of sediment-bound forms. For example, a greater mobilization of metal-contaminated sediments was observed on flood rather than ebb tides, implying that fine sediment fractions containing heavy metals may be retained within estuaries and that relief from exposure of estuarine biota to contamination may take considerable periods (Williamson et al., 1996). The western end of Princess Royal Harbour Albany, was closed to fishing because of lead and mercury contamination of sediments and biota from a fertilizer factory. Reductions in levels of heavy metals in tissues of estuarine organisms to safe levels for human consumption, took some ten years after discharges to the harbour ceased.
There is little known of the impacts of pesticides and other synthetic organics on Australian estuarine ecosystems and biota. Some investigations into levels in Australian biota from localised 'hot-spots' have been undertaken, but there have been no consistent investigations across various trophic levels, utilizing environmental gradients with different levels of exposure. There are a very large range of pesticides and other synthetic organic compounds of varying persistence and toxicity currently discharged to receiving environments (Richardson, 1995) and the effects of exposure of estuarine biota to these compounds are generally poorly understood. Some halogenated organic compounds such as organochlorine pesticides and poly-chlorinated biphenyls (PCBs) were valued because of their toxicity and persistence and were deliberately and carefully selected and manufactured to maximise these characteristics. Other synthetic organic compounds such as the polycyclic aromatic hydrocarbons (PAHs) and compounds such as dioxin, are the by-products of industrial processes, including hydrocarbon burning, chlorine bleaching and the chlorination of wastewater. Some of this later group of compounds are extremely toxic, mutagenic, bio-accumulate, bio-magnify and are highly persistent in receiving environments.
The three families of pesticides recently used widely in Australia include, in decreasing order of persistence; organochlorines, organophosphates and synthetic pyrethrums. There has been a phasing out of the organochlorine compounds in recent years because of concerns over bioaccumulation and bio-magnification and potential human health concerns. Organochlorines, once widely used in agriculture and for domestic pest control, are now tightly regulated and their inputs to Australian estuaries have declined significantly. Organochlorine pesticides and other halogenated organic compounds generally have very low solubilities in water, but are usually highly fat-soluble. Because of their persistence and fat solubility, these compounds have the capacity to bio-accumulate in the fat tissues of animals and plants up to 500,000 times greater than in surrounding waters (Richardson, 1995). Metabolism and degradation of organochlorine and other halogenated organic compounds produce a complex range of associated compounds or metabolites with varying persistence and toxicity.
Other pesticide compounds such as the organophosphates and the synthetic pyrethrums are being more widely used following the phasing out of the organochlorine compounds and they generally have much lower environmental persistence and lower acute and chronic toxicities compared to the organochlorine compounds. Potential problems may arise however, because these compounds have higher water solubilities and need more regular application than the organochlorines. They may also be highly toxic to non-target aquatic organisms. For example, synthetic pyrethrums have very low mammalian toxicities, but may be up to 1,000 times more toxic to aquatic crustaceans than are some organochlorine pesticides.
Synthetic organic compounds enter estuaries as either point or diffuse source pollutants and may be bound to soil or organic particles or dissolved in the water. Compounds with low water solubilities may be readily adsorbed to particles in the water column. Particles containing adsorbed synthetic organic compounds may flocculate and settle when they encounter increasing salinity, or be ingested by estuarine fauna. Zooplankton may actively seek and ingest detrital particles in the water column that contain adsorbed synthetic organic compounds and will either eliminate them in fecal pellets or store them. Suspension-feeding and deposit-feeding benthos may also ingest particles containing these compounds that have settled from the water column. Metabolism and elimination or retention of these compounds, depends on the nature of the compounds and on the metabolic processes in the animal. Bioturbation of surface sediments by benthic fauna plays an important role in the redistribution of synthetic organic compounds in estuarine sediments. The ecotoxicology of these compounds in the environment depends on their partitioning between the water column, sediments and pore waters and their tendency to be bio-accumulated and bio-magnified in estuarine biota. The impact on the biota depends on the nature and the duration of exposure and the life history and stage of growth of the organisms.
For the organochlorine lindane, organic matter in the sediments was found to be the primary sorption site in it's partitioning in sediment-water systems. The sediment-to-prawn bio-accumulation factor for Lindane was found to be 0.58, which meant that biotic concentrations would be significantly less than those observed in sediments (Just et al., 1990). Monitoring of estuarine and nearshore waters has found widespread but low-level contamination by organochlorine pesticides and their residues. Higher concentrations have been found where discharges arise from highly urbanised areas, or as runoff from intensive rural pursuits and results of some pesticides and their metabolites have frequently exceeded maximum residue limits (MRLs) set by the National Health and Medical Research Council (MHMRC), particularly for organisms higher in the food chain such as predatory fish (ANZECC, 1991).
There has not been widespread measurement of PCBs, dioxins or other halogenated organics in Australian surface and estuarine waters, probably because of the difficulty and expense associated with their accurate determination. A comprehensive investigation was undertaken in Port Phillip Bay during the late 1970s (Richardson and Waid, 1982), using sentinel mussels to determine the nature of PCB distribution in the Bay. PCBs were found in all of 87 samples of shellfish and in 27 sediment samples. Highest concentrations were adjacent to stormwater discharges from industrialised areas and lower concentrations were found remote from urban or industrial developments. Dioxins have been measured in some estuarine and nearshore locations adjacent to heavily industrialised centers and which have resulted in closure of fisheries in the area. Monitoring of these compounds in receiving waters has been poor, given their toxicity, persistence and presence on land (Richardson, 1995).
PCBs were detected in the abiotic and biotic components of the Brisbane River estuary with the highest concentrations in Pelicans and Gulls. For some benthic organisms (Capitella capitata) there was a direct relationship between sediment concentrations and body concentrations of PCBs (Shaw and Connell, 1982). There have been few investigations of PCBs, dioxins or other halogenated organics elsewhere in Australia and it may be speculated that the results for Port Phillip Bay were probably typical of other heavily industrialised and urbanised centers at that time. Improved pollution control provisions and management of industrial discharges have probably resulted in significant reductions in these compounds being discharged to the estuarine environments in recent times.
An additional class of synthetic organic compounds of concern in estuarine and nearshore waters are the metal-organics. Tributyltin (TBT) has been used in Australia as an active ingredient in marine antifouling paints since the early 1970s until its banning in 1988. It may still be used on vessels greater than 25m in length and so can still be detected in most ports and marinas where larger vessels berth.
TBT is highly toxic to marine fouling organisms and rapidly partitions to suspended sediments in the water column or the surface microlayer. TBT is moderately persistent in sediments with a half-life of around 3.5 years (Batley, 1995). In Cockburn Sound, Western Australia, levels of TBT in mussels (<0.8-732 µg TBT kg-1 wet weight) were above health criteria (WHO, 1990) for human consumption (146 µg TBT kg-1 wet weight) and the effects on biota themselves are little known. There are around 70 species of molluscs potentially at risk from TBT concentrations in the sediments of Cockburn Sound. Imposex (the induction of male reproductive organs in female animals) caused by TBT has been observed in molluscs in Perth metropolitan waters and indicate a significant impact of these compounds on mollusc reproductive strategies (EPA, 1990b; DEP, 1996).
TBT concentrations were measured in the Sydney rock oyster sampled from estuaries in NSW. Background TBT levels (<2 ng Sn g-1 dry weight), rose significantly (80-130 ng Sn g-1dry weight), where there were high boat densities and poor marine flushing (Batley et al., 1989). The concentrations of TBT in estuarine and nearshore sediments and biota have fallen since the banning of TBT for smaller pleasure craft and their occurrence is expected to fall in all areas other than major ports where larger vessels berth (Batley, 1995).
Both crude and refined petroleum products contain a wide range of chemical substances including alkanes, aromatic hydrocarbons and low levels of polycyclic aromatic hydrocarbons (Connell, 1995). Significant amounts of these compounds reach estuarine and nearshore waters continually through: direct spillage during unloading and refueling operations at ports, jetties and marinas; from outboard motors of pleasure craft (Yumlu, 1994); in stormwater runoff from urban areas from incomplete combustion of motor vehicle fuel or from spillage and; from minor amounts contained in sewage discharged to waterways (Connell, 1995).
Table 4.4 summarises some of the characteristics of common petroleum products and shows the relationship between number of carbon atoms (molecular weight) and boiling point. The boiling point provides an indication of the volatility and persistence of the compound. For example, low boiling point hydrocarbons like petrol, readily evaporate from the water and may completely dissipate within 24 hours of spillage. At the other extreme, asphalts and residual oils may persist in the environment for several decades (Connell, 1995).
Most hydrocarbons have very low solubilities in water and have higher lipid solubility. The alkanes generally have relatively low toxicities and the aromatic compounds are more toxic. The PAHs are at the extreme end of toxicities and are bio-accumulative and mutagenic, and have been implicated in human health problems and diseases in estuarine biota. The highest concentrations of PAHs were associated with sites adjacent to the most urbanised portion of the Brisbane River estuary (Kayal and Connell, 1989) and this is probably typical for other urbanised estuarine catchments.
| Table 4.4 Characteristics of some petroleum products . | |||
|---|---|---|---|
| Product | Types | Boiling point | Carbon atoms |
| Natural gas | alkanes | <20 | 1-6 |
| Petrol | alkanes and aromatics | 20-200 | 4-12 |
| Kerosene, Jet fuel and Diesel | alkanes and aromatics | 185-345 | 10-20 |
| Lubricating oils | alkanes | 345-540 | 18-45 |
| Asphalt and residual oils | complex aromatics | >540 | >40 |
Because of their low water solubilities and high affinity for lipids and organic compounds, hydrocarbons are normally found in organic-rich sediments. This may not occur in all cases however because the distribution of PAHs was not significantly influenced by organic carbon content and particle size composition of sediments in the Brisbane River estuary (Kayal and Connell, 1989). Minor oil spills are relatively common in estuarine and nearshore waters and the impacts of these episodic events have not been well studied. Minor oil spills have been found to cause damage through smothering to intertidal communities. The toxicities of surfactants used to remove oil from beaches and intertidal areas may be higher than that of the oil itself. Physical removal of oil, or leaving oiled areas to naturally clean themselves has become the preferred management strategy throughout Australia.
Investigations into waters, biota and sediments in estuarine and nearshore coastal areas throughout Australia have found widespread , but relatively low levels of PAHs mostly in the sediments and biota (various authors cited Connell, 1995). The mechanisms of uptake, metabolism and elimination by estuarine zooplankton and benthos are probably similar to those described for synthetic organics in the previous section. PAHs in the North Sea marine environment were found to have caused an increased frequency of DNA damage and physiological impairment in seastars (Everaarts and Sarkar, 1996).
It has been concluded that mature communities of organisms are resistant to invasion by introduced organisms, except when the evolutionary history of the community has not been exposed to the introduced organism (Cairns Jr. and Yongue Jr., 1973). This means that organisms introduced from overseas in ballast water have considerable potential to successfully invade local communities and become established. There have been numerous examples of introduced organisms becoming established in Australian estuarine waters and outcompeting indigenous communities. The tube worm, Sabella invading ports across the south of Australia and the Japanese starfish invading the Derwent estuary are obvious examples of introduced organisms having a significant impact on local benthic communities.
Less obvious, are introductions of potentially harmful dinoflagellates that have been associated with ballast discharges in Australian ports (Hutchings, 1992; Hallegraeff, 1995). Some of these organisms have the potential to cause shellfish poisoning and to severely disrupt shellfish aquacultural ventures. Fishing and recreational pressure can cause adverse impacts to benthic communities but the impacts are poorly understood and inconsistent. In a review of the impacts of trawling (Hutchings, 1990), investigations were cited which concluded that epibenthic communities were adversely impacted by trawling in nearshore areas, but there was no information on the impact of disruption of the epibenthos on infaunal communities or the food web generally. Otter prawn trawling was shown not to influence the abundance, species richness or diversity of epifaunal invertebrate communities in a sandy bottomed estuary in New South Wales (Gibbs et al., 1980).
Although data on recreational fishing throughout Australia are limited, it appears that anglers are the dominant harvesters of several species of estuarine fish species (West and Gordon, 1994). It was found that human predation through fishing and bait collection had a direct effect on the populations of organisms and an indirect effect on the structure of inshore assemblages of species along the New South Wales coastline (Kingsford et al., 1991).
Estimates of emissions from pleasure craft in Canada include 45,000 t yr-1 of hydrocarbons, 292,000 t yr-1 of CO, 13,000 t yr-1 of NOx and 2,500 t yr-1 of particulate material are added to the estuarine and nearshore waters (Yumlu, 1994). There is no information of this type available for Australian waterways, but the emissions on a per capita basis are probably comparable. It is difficult to determine the impact of these types of emissions on estuarine biota, but they may be significant in high use areas with low flushing. It would probably be difficult to isolate the impact of these emissions from other anthropogenic sources, because the highest usage of pleasure craft is adjacent to highly urbanised areas where there are significant diffuse and point source pollutant inputs.
It can be concluded that estuaries are highly dynamic and variable ecosystems. There is a large range of natural and anthropogenic stressors that can impact on the abundance, distribution and community composition of estuarine biota. In such a variable world, there is a requirement for statistical rigor in the assessment of impacts and response in spatially and temporally variable communities (Underwood, 1991; Morrisey et al., 1992a; Morrisey et al., 1992b; Underwood, 1993; Kirkman, 1996; MacIntyre et al., 1996). Any interpretation about the role of human disturbance may be confused with any natural, inherent cause of variability between locations in the time-course of mean abundances of the organisms being used to monitor the impact of the disturbance.
In addition to changes in average conditions, natural and anthropogenic disturbance may also cause:
Assessment of environmental change should therefore not be preoccupied with changes in the averages of some variable alone. The natural variability of a population in the undisturbed state should be assessed prior to or remote from the disturbance, in order to provide an appropriate baseline against which data collected after, or at the site of disturbance, can be compared. There is considerable danger in under-representing the number of control samples required spatially and temporally (Underwood, 1991). As an example of the danger of inconsistent sampling: because sampling design varied with each study, it was not possible to detect significant changes in the nutrient status of Port Phillip Bay during the fifteen years from 1975 to 1990 (Longmore, 1992), even though there had been significant increases in nutrient loads to the system over the same time period. Inconsistent sample design meant that the impacts of changing pollutant loads on estuarine biota over decades could not be determined reliably (Patrick and Palavage, 1994).
Disturbances usually cause impacts both spatially and temporally and should thus be sampled with nested designs. Temporal replication should include several time-scales, one longer than the other, with random times within each of the periods and should take account of the known biology of the organisms being monitored (Underwood, 1991; Underwood, 1993). Random, not regular sampling designs are less likely to exactly coincide with some cyclic pattern and thereby cause some problems of interpretation of the data. Temporal samples should be independent of one another and take account of serial correlation (Gilbert, 1987).
Choice of spatial scale is also important. For example, inconsistent responses by soft-sediment biota were observed between experimental plots 10 m apart and between replicate experimental sites more than 100 m apart (Morrisey et al., 1996). Consequently the choice of spatial scale should be determined and justified in terms of the processes operating and the dispersal and dispersion of the population being sampled (Underwood, 1993). Whatever spatial scales are chosen, there should be sufficient replication to determine differences between undisturbed and disturbed locations and spatial correlation should be taken into consideration (Gilbert, 1987).
Even with 'good' sampling design, inconsistent results are common. Sources of uncertainty in sampling programs include: insufficient numbers of sampling sites and replicates (Fairweather, 1991; Underwood, 1991; Underwood, 1993); insufficient temporal coverage (Maher et al., 1994); absence of study of some important aspects of the environment (Martin and Richardson, 1991); lack of consistency in sites studied over time (Longmore, 1992; Patrick and Palavage, 1994); inadequate commitment to continue monitoring (Young, 1995); changes in political direction; and inadequate opportunities for related research and publication in the international literature (Martin and Richardson, 1991).
Monitoring and sampling methods may also produce uncertainty in observations. Different sampling methods (sweep net > net > corer), produced different results for species richness in mobile benthos and smaller 50 mm cores were found to be better for sampling infauna than 150 mm cores (Edgar et al., 1994). Recovery of macro-benthos was influenced by mesh size with 0.25 mm sieve recovering all macrofauna adequately, but only 86% of biomass and 55% of numbers being recovered from 0.5mm mesh and 49% of biomass and a mere 8% of numbers recovered from 1.0 mm mesh (Schlacher and Wooldridge, 1996).
There are a great range of sampling and sorting methods available for stream invertebrates which may influence the final estimates of species richness and abundance (Chessman, 1995; Walsh, 1997). Investigations into the relationships between sorting strategy and statistical significance of between-site and within-site differences revealed that several non-abundant taxa were important contributors to differences between treatments (Walsh, 1997).
Care should also be taken in extrapolating laboratory findings to the field (Thrush et al., 1996). For example, there were distinct differences between laboratory-based and field relationships in the partitioning of PAHs in the Brisbane River estuary (Kayal and Connell, 1990). Considerable stochastic variability was observed in mesocosms used to test the impacts of chlorpyrifos and nutrient enrichment on oligochaete communities when the mesocosms were in an early stage (Verdonschot and Ter Braak, 1994)
There were significant increases in photosynthetic pigments, species richness and a shift from episammic to periphytic diatoms in mesocosms compared to field sites where sediment collections were made (Kendrick et al., 1996). Bioassays are imperfect tools that cannot completely replicate the in situ light and temperature fields: they restrict processes that recycle nutrients and frequently contaminate samples with trace metals. Bioassay results should therefore be interpreted conservatively by considering only the largest differences and most consistent patterns (Thompson and Hosja, 1996).
Data analysis and interpretation of results may lead to uncertainty. Some software used to investigate between-site differences provides the option to exclude rare species from the analysis. This may serve to improve stress levels or the 'goodness of fit', but this practice may also reduce discriminatory power by removing subtle nuances conveyed by the rarer species. Non-parametric multivariate techniques have been used to assess changes in community structure. These may be undertaken within a framework consisting of i) display of spatial patterns through ordination and clustering, ii) identification of species strongly influencing groupings, iii) statistical tests for differences in space and time and iv) the linking of community differences to key physico-chemical environmental variables (Warwick, 1986; Clarke, 1993; Warwick, 1993).
Ascribing the cause of an observed pattern of change or difference to a particular disturbance is very difficult with multivariate analysis, and univariate measures of abundance of a particular species, may provide useful information on causal links (Underwood, 1993). There are numerous univariate measures of community structure and they may all have certain disadvantages (Pielou, 1975) and may be highly correlated (De Benedictis, 1973). Biomass-based measures of community structure showed different patterns to frequency-based measures, but they were of uncertain ecological significance (Rainer, 1981).
Recent studies (Warwick, 1988; Gray et al., 1990; Warwick, 1993; Somerfield and Clarke, 1995; Chapman, 1998) have indicated that patterns of anthropogenic disturbance may be as readily detected at coarse taxonomic resolution as could be observed using species data. Little difference in the perceived pattern of impact was observed when species data were aggregated to the level of genus, although distortions in patterns were observed at coarser levels of taxonomic resolution. It was suggested that if the pattern of community change was marked, interpretable results could be observed for all levels of taxonomic resolution even to the level of phyla (Somerfield and Clarke, 1995). It has been tentatively concluded that anthropogenic effects modify community composition at a higher taxonomic level than natural environmental variables which influence fauna more by species replacement (Warwick, 1988; Warwick, 1993). This phenomenon may be more pronounced for severe disturbance or for xenobiotic agents than for disturbance by nutrient enrichment or organic loading (Patrick and Palavage, 1994).
These results have ramifications for the costs and data requirements for detecting anthropogenic disturbance.
Estuaries are highly dynamic ecosystems with considerable spatial and temporal variation in sediment and water column characteristics. Accordingly, considerable variability in the distribution of estuarine biota has been observed at spatial scales from metres to kilometres and for temporal scales from days to months (Morrisey et al., 1992a; Morrisey et al., 1992b). Additionally, localized disturbances caused by runoff events, currents, wave action and other disturbances, may act to physically disrupt benthic communities in estuaries and reset them to less mature states with reduced species diversity, size strata and stability (Johnson, 1970). One of the few common characteristics of estuarine biota, is that their distributions can be described as a spatial and temporal mosaic (Johnson, 1972; Neale and Bayly, 1974).
There are significant problems associated with determining the nature of anthropogenic stressors in naturally heterogeneous environments such as Australian estuaries (Thornton, 1987; Underwood, 1991; Underwood, 1993), particularly where natural variability and disturbances act to maintain benthic communities in a patchwork mosaic (Rainer and Fitzhardinge, 1981; Gray and Pearson, 1982). The impacts of toxicants on biota may be more obvious than those of organic or nutrient loadings (Patrick and Palavage, 1994), although assessing the impacts of toxicants on biota is not without difficulty. Seasonal variations in species composition of estuarine communities, differences in response to exposure by organisms at different stages of development (Tomlinson et al., 1980) and potential synergistic and compounding effects of pollutant cocktails, all serve to confound assessments of estuarine ecosystem health.
In addition to the complexities described above, the concept of ecosystem health itself has well recognised problems of application and interpretation, even though it has now become established in mainstream management literature. Ecosystems exist as nested sets of linked process-functions with temporal boundaries, not as tangible superorganisms with spatial boundaries (Callicott, 1995). Environmental indicators need to take account of these interactions.
The following quote provides a cautionary note:
Ecosystems are complex systems, which means that their behavior cannot be simply explained by deconstruction to individuals, to species, or to communities and then describing the responses of individual elements. Accordingly, no single measure or group of measures will provide a perfect view of ecosystem processes contributing to ecological health and inconsistencies may regularly confound health assessments (Fairweather, 1997). Ecosystem health itself is not easily measured, but measures correlated to health are available.
In freshwater and marine systems, there are numerous indicators and biological pollution indices, which have received widespread scientific support and are currently in widespread use as management tools. Indices are required for estuaries to aid in communication, integration and interpretation. Scientists may mistrust indices because the clarity of the original data may be blurred by indexing procedures, but it has been recognised that the potential advantages for communication of estuarine threats and degradation to non-specialists outweigh any loss of scientific credibility. Indicators should therefore be seen not as a replacement for careful consideration of multidimensional information by practicing professionals, but as an adjunct to assist in a wider appreciation of complex non-linear behaviors.
A review of indicators and indices of estuarine nutrient enrichment described the following index methods which had been used up to that time (McErlean, 1981):
More recently, Agenda 21 and national State of the Environment (SOE) reporting have called for the development of indicators of sustainability and of ecosystem condition, of pressures on ecosystems and for policy responses to pressures on ecosystems (OECD, 1994; ANZECC, 1998; Fairweather and Napier, 1998; Ward et al., 1998a; Ward et al., 1998b). National adoption of the pressure, state, response (PSR), model for SOE reporting has suggested numerous environmental indicators which could be used.
The USEPA have developed an environmental monitoring and assessment program (EMAP), for near coastal waters and estuaries (Holland, 1990), which uses a similar approach to that suggested by the OECD. The EMAP indicator set includes; response indicators, habitat indicators, exposure indicators and research indicators. Unlike the ANZECC recommendations for indicators in Australian environments, which provide only broad guidance, the USEPA provided detailed protocols for the application and interpretation of environmental indicators including:
There are several important differences that preclude an early adoption of the USEPA protocol for Australian estuaries. Firstly, much is known about the distribution and dynamics of estuarine biota in the USA. Secondly, much is known of the impacts of contaminants on the distribution and abundance of estuarine biota and thirdly, there is considerably less seasonal heterogeneity in salinity fields and other constituent distributions in USA estuaries, which means their systems are somewhat easier to investigate. Finally, the USEPA and NOAA had jointly procured funding to provide for consistent national monitoring and reporting.
The following sections of this Chapter discuss the application of bio-indicators used to describe aspects of ecosystem health. The tables in Appendix 1, provide examples of the global application of environmental indicators for estuaries. Some indication of the scales of application, data requirements, advantages and disadvantages, potential application and costs are provided. Potential application includes an assessment of whether the particular indicator is: core, in current use; development, where links between response and indicator criteria have been established, but require further development for implementation and; research, where links and bounding conditions are yet to be described.
The tables in Appendix 2, provide examples of biological indicators used to describe aspects of ecological health in Australian estuaries.
The number of potential ecological indicators is infinite and accordingly, a large number of indicators appear in the literature.Indicators can be broadly grouped into three types:
While the first two classes of indicators may have a solid scientific base, socio-economic indicators are somewhat more subjective. For example, human population of a catchment, aesthetic considerations, or rates of recreation for a particular ecosystem may correlate with environmental stress, but these measures provide little diagnostic information for managers and policy makers, especially in the context of a globally increasing population.
Every measurable parameter has some value with regard to assessing environmental conditions. However, measuring every environmental variable or assimilating a large amount of information into the decision making process is not possible. Environmental parameters or indicators must be selected that are useful in judging the degree to which specified environmental conditions have been achieved or maintained.
An ecological indicator is a characteristic of the environment that, when measured, quantifies the magnitude of stress, habitat characteristics, degree of exposure to the stressor, or degree of ecological response to the exposure (Hunsaker and Carpenter, 1990). Indicators must be related to management, which will of course embody current interpretations of social and biological relevance. The spatial and temporal scales of the endpoints of monitoring will be dictated by the management goals.
The search for measures that will uniquely indicate the effects of pollution or any other deleterious change in the environment of a community has been longstanding. Biological responses tend to integrate the independent and interactive effects of many stressors, a property that makes them potentially more robust indicators of ecosystem condition than the concentrations and loadings of individual chemicals (Cairns Jr. et al., 1993; Clarke, 1994). There is a requirement for experimentation which identifies indicator variables which are causally linked to human impacts (Keough and Quinn, 1991; Fairweather, 1997).
There are a myriad of biological indicators published in the literature, particularly in recent years as the limitations of physico-chemical indicators become known and it was felt that waterway managers required less ambiguous measures of the ability of ecosystems to sustain themselves and their biota. Biological indicators may be described as a hierarchy from measures of i) ecosystem function, ii) community structure, iii) biotic indices, iv) indicator species, v) biomarkers and vi) combinations of these. Clearly each of the various types of biological indicators describe processes at different spatial and temporal scales and perform different functions as indicators of environmental stress. Although care should be taken in the use of these indicators because organisms and communities may respond differently to different types of stressors it is useful to examine this heirarchy further:
The broader objectives of ecological management, such as the restoration of self-maintaining ecosystems, can be framed in both structural and functional terms. Decisions regarding the use of structural versus functional indicators of ecosystem condition must be based on the goals and objectives of ecosystem management programs, the types of stressors present and the nature of the biological communities present. There have been significantly fewer investigations into these types of dynamic processes than there have for static inventories (see counts of references by category in Tables A2.1 - A 2.3 in Appendix 2). Investigations into functional aspects of ecosystems require a greater understanding of the history of the site and an understanding of the dynamics of physical and biological processes than do inventories (Fairweather, 1997).
Theoretical ecologists have used community transition graphs to define interactions at the ecosystem level (Luh and Pimm, 1993). This technique has been suggested as being applicable for defining the success of restoration ecological studies and for detecting the level of maturity of natural and impacted communities. These techniques may have difficulty in application in seasonally heterogeneous systems such as Australian estuaries. Analysis of food web dynamics (Havens et al., 1996a) or ecosystem production and respiration ratios (Niederlehner and Cairns Jr., 1994), provides a means of integrating various observed direct and indirect impacts of stressors on different communities into a broader management perspective. Food web dynamics are the basis for most aspects of ecosystem operation and can be directly related to ecosystem integrity (Cairns Jr. et al., 1993; Carpenter et al., 1995).
Whole ecosystem experiments have been carried out in North America to define ecosystem stability and function. In one experiment, lake acidification eliminated fish reproduction at pH 6.0 and sensitive invertebrate communities at pH 5.6 to 6.1, but primary production was unaffected even at pH 4.5 (Carpenter et al., 1995). This sort of information highlights the complexity of interactions and is particularly relevant to indicator development. Productivity of the benthos was used as an indicator of the health of Gunnamatta Bay (NSW); lower relative production compared to less-disturbed estuaries may have been caused by non-specific effects of urban encroachment (Rainer, 1982). These results are encouraging but indicate that causal links need to be established before these types of assessments could be use routinely.
Fairweather (1997) has advocated the use of "ecoassays" to explore relationships between stressors and ecological processes in estuaries. Investigating processes provides insight into the dynamic nature of ecosystems and how biological communities might respond to stressors. Processes that lend themselves to ecoassay include rates of; recolonisation, reproduction, carnivory, herbivory and bioaccumulation. Rates of algal colonization and shipworm attack on wooden blocks were used to investigate ecosystem health of estuarine mangrove communities in New South Wales. Algal colonization provided good discrimination at three spatial scales while shipworm attack was not significant between or within estuarine sites (Fairweather, 1997). Most of these processes are the net effect of multiple interactions between organisms and the abiotic environment.
Ecoassays may be of use in seasonally heterogeneous Australian estuaries by assigning key "index periods" (Holland, 1990) in which to focus efforts. Index periods could be selected to coincide with episodic events or periods of particular activity of the organisms of interest, such as following floods (Saenger et al., 1980) or reproductive cycles. Thus by focussing efforts on key rates and processes and at key index periods, much could be learned about the dynamic nature of biological response to natural and anthropogenic stressors.
Unfortunately, it is considered that understanding ecosystem function to the extent that adverse or beneficial changes can be described through ecoassays is still some way off, particularly for estuaries in Australia (Fairweather, 1997).
There are numerous indices available to examine community structure. All measure aspects of the abundance and species richness of communities, but they are often highly correlated, suggesting that any overall trends demonstrated with one set of measures will also be demonstrated with another set (De Benedictis, 1973).
Analysis of community structure can be roughly divided into univariate methods (e.g. diversity indices), graphical methods, from which numerical indices can be derived visually, and multivariate methods, which have excellent powers of integration, to a lesser extent interpretation, but not as yet for easy communication (Wilson, 1994).
Although grounded in ecological theory, many of these measures have questionable applicability to real world situations. Calculating statistics that describe numbers of organisms do little to improve understanding of the dynamic nature of the interactions between species (Pielou, 1975). Many of these measures are accompanied by spatial and temporal heterogeneity and require robust sample designs to be meaningful (Underwood, 1993). It was observed that there was a hierarchy of heterogeneity in the estuarine benthos recovering after a severe flood in the Calliope River (Qld) where abundance>species richness>diversity>evenness (Saenger et al., 1980).
The usual community-level measures (species richness, diversity), generally have questionable theoretical justification, have few documented cases of causal links between stress and response and require a high level of taxonomic precision. Where there has been only mild stress, indicators of community structure may be of limited usefulness (Rainer, 1981).
Determination of species richness involves the assessment of the number of species present at a particular place time standardised by abundance. The inference for health determinations using species richness is that a greater number of individual species translates to a greater resilience and stability of the biotic community. It has been found that communities experiencing 'mild disturbance' what ever the cause, have a greater number of species than undisturbed or heavily impacted sites (Connell, 1978; Gray, 1992).
Availability and diversity of food supplies were also found to influence species richness of benthic communities in seagrasses (Bell et al., 1988; Edgar, 1990a; Edgar, 1990b), and these types of interactions confounded assessments of the impact of anthropogenic stressors on species richness. Measures of species richness were used to compare long-term changes at a sewage outfall , but there was considerable heterogeneity in the data (Brown et al., 1990).
In some freshwater streams, consideration of species richness alone may provide a more accurate assessment of stress-related changes in natural communities than the use of diversity indices alone (Cairns Jr. et al., 1988). This may not apply to Australian estuaries, where species richness was found to be more variable than diversity measures (Saenger et al., 1980). It has been established that anthropogenic stress may impact on species richness (Rapport et al., 1985), but the potential bidirectional nature of the response with increasing levels of stress confound the use of this indicator. An additional disadvantage of species richness is the need to identify and count rare species and consequently a greater level of taxonomic resolution is required, when compared to data for calculating diversity indices, where relative abundance of the more common species is more important than identifying and counting rarer species.
There may be little diagnostic precision gained from the assessment of the rare fraction of estuarine communities. For example, in a North Sea benthic community, changes in the occurrence of rare species from mostly present to mostly absent, were observed adjacent to oil production platforms. It was only under severe pollution that ratios of opportunistic species changed (Gray et al., 1990). Long counting times required for accurate determinations of species richness, the influences of food supply and habitat diversity, together with bidirectional response with increasing anthropogenic stress makes this measure impractical as a routinely monitored indicator, however it continues to be one of most commonly described indicators (see counts for published papers by category in Appendix 2), but usually without appropriate critical analysis of its utility.
Relative abundance has been the most commonly assessed indicator in estuaries. Estimates of biomass, density, cover or standing crop of estuarine communities have been used to describe the nature of estuaries. Absolute values do not in themselves provide an assessment of health, but relative changes may provide a coarse indicator of community change related to environmental stress. Phytoplankton standing crop (chlorophyll-a) has been used as a measure of trophic conditions (Hunsaker and Carpenter, 1990; Burford et al., 1995) and macroalgal biomass has been used as a measure of eutrophication in the Peel-Harvey estuary (Lavery et al., 1991) and Princess Royal Harbour. Changes in seagrass cover and leaf detrital biomass were used as a measure of ecosystem health in Cockburn Sound (Western Australia). From 1954 to 1978 the meadow reduced from an area of 4,200 ha to 900 ha or from an annual leaf detrital production of 23,000 t to 4,000 t (Cambridge and McComb, 1984).
Measures of abundance or cover within trophic groups do not in themselves provide information on causal links when assessing ecological health. Abundance or biomass comparisons across trophic groups however provide considerable insight into potential interactions between organisms. Counts or relative biomass of predators or prey (food) and their relationship to the organism of interest, provide information on energy flows in ecosystems. Abundances of benthos in NSW estuaries varied considerably with time but there was little syncrony in variations in abundance among species at different sites or even among the species at a single site. The lack of syncrony in sample statistics was interpreted as meaning that the changes in physical conditions were unimportant to the community as a whole (Rainer, 1981).
As with species richness, there are a number of natural stressors which influence abundance, particularly in estuaries. In the light of significant natural variation in abundances of estuarine organisms (Brown et al., 1990), it may be difficult to use abundances alone to determine ecological health. Biomass measures alone rarely provide adequate information for assessing trophic conditions, although estimates may be useful as part of a combined trophic index. Abundance continues to be one of the most widely reported indicators in the literature (see counts for published papers by category in Appendix 2). Abundance/biomass or K-dominance curves, (Clarke and Warwick, 1994), have been successfully used to identify shifts in benthic community structure to larger numbers of smaller opportunists (DEP, 1996) and offer potential as indicators of change or a means of spatial comparison.
Biodiversity may be defined as both the total number of species in a community and their relative abundance. Diverse communities are defined as those having a large number of species with an even distribution of abundances (Harris, 1994). The ability to combine information on species richness and relative abundance in a single measure explains, in part, the appeal of these measures. Theoretical relationships between diversity and stability or resilience have led to the widespread belief that high species diversity is an attribute of a healthy ecosystem. This may in fact demonstrate a community experiencing mild stress or the first stages of more serious disturbance (Connell, 1978).
Measures of diversity are relatively simple to estimate because individual taxa need not be identified fully. Often the level of assessment of what constitutes a separate species and not a life stage or morphotype of a particular species is sufficient. Less taxonomic rigor is required and because the relative abundance is used, more rapid counting is possible with less emphasis on the rarer species. A disadvantage of diversity indices is that the identity of individual species is ignored, so the measures are not sensitive to compensatory changes in the community when one dominant species is replaced by another dominant species (i.e. no change in diversity).
The most widely used diversity measure has been the Shannon-Weiner index (Shannon and Weaver, 1949) expressed as;
H' = - S pi log pi (Where: pi is the count for the ith species).
This index is the only mathematical function that ensures that an even community has a greater index than an uneven one with the same number of species and a community with a greater number of species than another will have a larger index where the evenness of both communities is the same.Because of the second property, H' should be standardised for comparisons between communities of different numbers of species.Standardised H' of Pielou's equitability is given as:
J' = H' / (log s) (Where: s = the number of species).
The Pearson diversity index has been used to describe biotic communities and this measure is simply the number of species which contain at least 10% of the total number of organisms in the sample (Pearson et. al. 1967 cited Neale and Bayly, 1974). This simple index showed differences in the diversity of zooplankton communities in four Victorian estuaries. The estuary having the greatest numbers of marine species in addition to estuarine species had a higher Pearson Index than other estuaries with predominately estuarine species (Neale and Bayly, 1974).
Diversity at the local level may be influenced by a range of biological and physico-chemical factors including the recent evolutionary history of the community (Harris, 1994). Diversity was found to be more predictable for highly diverse communities with no dominant species than for communities with low diversity dominated at all times by one or two species (Poore and Kudenov, 1978b). Diversity of the Yarra community was low compared to elsewhere and indicated that the river was in poor condition (Poore and Kudenov, 1978b). Biomass-based measures of diversity and evenness indicated patterns that were different from frequency-based measures (Rainer, 1981) but the implications of these differences were not clear. Diversity and evenness could not be interpreted as indicators of environmental harshness in a benthic community (Rainer and Fitzhardinge, 1981).
Despite questionable theoretical justification, variability in observed index values and uncertainty in interpretation, diversity measures remain the most commonly expressed indicators of community structure. Pielou (1975) concluded that diversity indices should not be overvalued and that determining the structure of a community entails more than calculating its diversity index.
The relative size distribution of biotic communities has been widely used to describe the level of stress. For benthic macro-invertebrate communities where large organic loads have occurred, there appears to be a consistent loss of larger species in favour of smaller opportunistic species that have a greater tolerance to reduced dissolved oxygen levels (Poore and Rainer, 1974; Poore and Rainer, 1979; Gray and Pearson, 1982; Warwick, 1986; Gray et al., 1990). Similar conclusions have been made about plankton communities but the evidence is not conclusive (Harris, 1994). Dominance by microplankton and nanoplankton in the Parramatta estuary followed extended cultural nutrient enrichment (Revelante and Gilmartin, 1978). It has been observed that for inland waters, increased nutrient availability may result in larger species of phytoplankton becoming dominant (Harris, 1994) or phytoplankton communities both increasing and decreasing in biovolume irrespective of nutrient availability (Cichra et al., 1995), but it is not clear whether this phenomenon occurs in estuaries.
Zooplankton may influence the size classes of phytoplankton by preferentially grazing on smaller diatoms (Fahnenstiel et al., 1995) or by actively avoiding certain less palatable species (cyanophytes) (Paerl, 1988). A significant change in the body length of zooplankton over several years was related to grazing pressure by higher order consumers, the size distribution returned to normal following the removal of predators (Moss et al., 1996). Grazing pressure by a marine zooplankton grazer was suggested as having precluded a euryhaline zooplankton species from the lower reaches of the Swan River estuary (Hodgkin and Rippingale, 1971).
Increased organic loading, nutrient supply or fine sediment to estuaries has the potential to increase fine particles in sediments and thus resuspension. Benthic macrophyte communities may be adversely impacted by light limitation through higher turbidity from increased sediment loads or increased phytoplankton activity following nutrient enrichment. Increased epiphytic growth on benthic macrophyte communities has also resulted in a reduction in seagrass cover. Reductions in seagrass and macroalgal turf have resulted in increased sediment resuspension in estuaries. This may favour smaller deposit-feeding macrobenthos over larger suspension-feeders.
The net effect of increased nutrient, sediment or organic loadings to estuaries may be a shift to smaller, opportunistic deposit-feeding macrobenthos. Changes in the size strata of benthic macrofaunal communities may thus be a reliable indicator of disturbance. Transects around sewage outfalls have confirmed these trends and have also revealed the effect was less pronounced where there was less disturbance some distance from the outfalls. It is likely that disturbance-induced reductions in the size strata of macrobenthos may only occur after considerable environmental damage has occurred and thus these types of indicators offer little early-warning or diagnostic precision.
The establishment of large introduced suspension-feeding organisms such as the polychaete Sabella, may confound these relationships. Sabellid polychaetes, through their sheer size and numbers, have the ability to exclude deposit-feeders. Deposit-feeders play an important role in bioturbating sediments and acting as a sink for N, through their enhancement of the eflux of NO3–N and N2 from sediments. Establishment of Sabella and the loss of deposit-feeding macrobenthos may lead to an increase in NH4–N eflux from sediments thus favoring phytoplankton communities (Nielsen and Jernakoff, 1996). In this instance, reduced ecological health of a marine embayment of estuary would be accompanied by an increase in the size strata of macrobenthos (Sabella).
Biotic indices summarize the responses of several indicator species to stress, so as to provide a single number that characterizes ecosystem condition. Species vary in their response to different stressors and consequently biotic indices are of most use in situations subject to single stressors, which may of course occur only rarely. Indices of biotic integrity (or 'metrics') are increasingly being used to assess and monitor ecosystem health globally, particularly using benthic macro-invertebrate communities. Three general types of metrics have been described (Cairns Jr. et al., 1993);
Macro-invertebrate indicators of biotic integrity are generally based on taxon richness assessed at various levels of taxonomic resolution, the proportion of tolerant species and the dominance of different feeding groups (Wilson, 1994). Macro-invertebrate indices developed for one particular situation may need modification to be applicable in a different situation, particularly the various weightings placed on each of the elements of the combined metric.
In combined form however, metrics of this type offer little diagnostic ability and have questionable theoretical basis. Multivariate techniques, including principal components analysis, have been used to select appropriate weightings for combined metrics (Davis and Lubkin, 1989). A simple linear combination of Shannon-Weiner diversity index, the proportion of total benthic abundance as tubificid oligochaetes and the proportion of total benthic abundance as bivalve molluscs has been successfully used to classify polluted sites in the Gulf of Mexico (Engle et al., 1994).
A benthic macro-invertebrate index has been developed as part of the USEPA's EMAP program, that combines a salinity normalized measure of expected species richness (Gleason's D), salinity normalized expected number of tubificid oligochaetes and the abundance of spionid oligochaetes (Strobel et al., 1995).
| Benthic Index score = | 1.389 (% expected Gleason's D - 5.15) / 28.4 |
| - 0.651 (normalized tubificid abundance - 28.2) / 119.5 | |
| - 0.375 (spionid abundance - 20.0 ) 45.4 | |
| where: | |
| % expected Gleason's D = | Gleason / (4.283-0.498 * bottom salinity |
| + 0.0542 * bottom salinity2 | |
| - 0.00103 * bottom salinity3) * 100 | |
| Normalized tubificid abundance = | Tubificids - 500 * e-15* bottom salinity |
The above formula was included here to illustrate the degree of sophistication and complexity of weightings used when combining elements in many biotic indices. Indices of this type would clearly have limited applicability outside areas where they were developed and calibrated. These types of indicators require a detailed understanding of community structure and the changes occurring following disturbance and as such have the potential to provide considerable discriminatory power for a local situation when calibrated. Biotic indices also require detailed knowledge of the autecologies of individual species or of members of functional groups to be combined.
There has been little published work on biotic indices in Australian estuaries. There needs to be considerable additional work to be undertaken before these types of index become available for routine screening of ecological health, particular for Australian estuaries with significant seasonal heterogeneity.
The development of indicator species has centered on the identification of surrogate organisms that integrate critical physical, chemical and biological properties of the ecosystem and thus can be used to judge the relative health of the ecosystem as a whole (Cairns Jr. et al., 1993). In general terms, a group of organisms with known autecologies probably yields a greater level of understanding than a single organism (Whitton and Kelly, 1995).
In river monitoring, and probably also for estuaries, ideally the organism or group of organisms to be used should; i) be present throughout the length of a river, ii) should grow in a specific well defined habitat, iii) should be easily sampled and present in abundance, iv) should be unaffected by life cycle stages and v) should react to changes in water quality so that specific groups can be used as markers (Round, 1991; Whitton, 1991). Presence rather than absence in response to environmental stimulus is a preferred characteristic for indicator organisms.
In an investigation of the success of the Clean Waters Act in the USA, Patrick (1994), found that because of the inconsistencies in data collection, analysis and reporting, published accounts of interactions between biotic communities and physico-chemical indicators could not be used to define trends over the longer term. The presence of indicator species and groups of species were believed to be less influenced by variations in sampling methods than if measures of community structure were used. In this investigation (Patrick and Palavage, 1994), found that algae and macro-invertebrates provided better indicators of xenobiotic contamination than fish, although no group of organisms provided good discrimination in an estuary enriched with agricultural runoff.
Indicator species have been used to screen waterways for potentially harmful phytoplankton (Hosja and Deeley, 1993). Here the presence of cyanophytes or dinophytes (red tides) and other potentially harmful organisms (e.g. PSP, NSP organisms), were regularly screened and the potential for harm assessed.
Indicator species have the potential to provide considerable information on particular nuances of local environments. There needs to be a detailed understanding of the autecology of indicator species, such that the interactions with natural cycles, anthropogenic stress and other biotic interactions can be described and taken into account during health assessments.
A wide range of sublethal responses for various aquatic organisms have been used to assess anthropogenic stress. These include physiological responses such as changes in growth rates, fecundity, oxygen consumption, enzymatic reactions, structural changes and genetic aberrations (Nowak et al., 1993; Clarke, 1994; Holdway et al., 1995).
Bile metabolites, RNA/DNA ratios, adenylate energy charge, skeletal abnormalities, immune dysfunction, behavioral changes and histopathological lesions have been cited as examples of biomarkers for use in detecting the impacts of anthropogenic stress through exposure to xenobiotics (various authors cited, Holdway et al., 1995). The sterol marker series has been used to identify the possible sources of organic matter inputs to a temperate estuary, including that from higher terrestrial plants, freshwater and marine phytoplankton and sewage (Laureillard and Saliot, 1993).
Sublethal biomarkers that have been evaluated in the literature include the presence of mixed-function oxidase in fish exposed to xenobiotics in Port Philip Bay (Holdway et al., 1995), DNA damage in seastars (Everaarts and Sarkar, 1996) and chromosomal aberrations in developing fish eggs (Klumpp and von Westerhagen, 1995).
Coprostanol, a fecal sterol biomarker, provided a good indicator of sewage inputs (Nichols and Espey, 1991; Nichols and Leeming, 1991) and potential thermotolerant coliform contamination of waterways. Concentrations of 60 and 400 ng l-1 of coprostanol were found to be broadly equivalent to existing bacterial standards defining primary and secondary contact limits (Leeming and Nichols, 1996). Sterol biomarkers showed that the mid estuary and parts of the upper Derwent estuary were severely contaminated by sewage and the upper estuary was contaminated by pulp fibre from a paper plant (Leeming and Nichols, 1998).
There are two basic measures of developmental stability (Clarke, 1994; Klumpp and von Westerhagen, 1995; Tracy and Hough, 1995);
The assumption with these types of assessments is that organisms are normally buffered against producing morphological deviants and under stressful conditions the buffering mechanisms are reduced. This means that there may be a greater occurrence of these types of aberrations when populations of organisms encounter stress. Because of the possibility of low levels of these deviations in non-stressed communities, these types of assessments need to use 'before-after or control-impact' experimental designs in order to compare disturbed systems to the normal level of developmental instability in reference communities (Clarke, 1994).
Contamination of biota by TBT was found to lead to developmental instability manifesting itself as shell deformities and reduced tissue weights in estuarine oysters (Batley et al., 1989). Body burdens of TBT in Port Phillip Bay were low (<10 ng g-1 Sn, as TBT wet wt) and were not related to imposex indices in gastropods (Foale, 1993). Transplanted gastropods failed to develop higher frequency or intensity deformations than controls, suggesting that imposex may be irreversible and that the higher frequency of occurrence of imposex in the native population, may be related to historical TBT exposure. Transplants may be useful in investigating the current exposure (Foale, 1993).
Developmental stability analyses and biomarkers have the potential to offer a degree of early warning of environmental change, because sublethal effects may be detected before serious environmental impact has occurred. Indicators of this type offer a high degree of discriminatory power when calibrated for a particular situation. Potential disadvantages of these types of bio-indicators are differential susceptibility across a range of organisms and the need for detailed histopathological investigations to establish these indicators. Sub lethal effects such as these are not useful for detecting the impacts of organic enrichment or nutrient enrichment.
The USEPA has recently developed a non-taxonomic measure of nutrient availability and eutrophication called a trophic state index (Hunsaker and Carpenter, 1990). This index combined measures of chlorophyll-a, water clarity, N and P concentrations and is relatively easy to measure. It is unclear whether this index without modification would be suitable for estuaries in Australia because of significant seasonal heterogeneity and the presence of tannins and dissolved organic carbon compounds, which may influence the relationships between water clarity and phytoplankton biomass. Naturally oligotrophic lakes (Bowling, 1988; Wrigley et al., 1988) and estuaries like Broke Inlet in the southwest of WA, and in Tasmania (Edgar et al., 1998) are naturally very dark in colour, are crystal clear and may have Secchi depths of < 2 m.
The United Kingdom currently uses an estuarine health index called the National Water Council index to describe estuarine health (Wilson and Jeffrey, 1994). This index combines DO status, biological quality of fish habitat and benthos with observable pollution. Data requirements include DO data, an assessment of migratory fish passage and resident fish diversity and benthos, toxic or tainting substances, pollutant inputs and nature of detectable impacts. These indicators are given a weighting and combined to provide a value for an estuarine segment of whole estuary. The index is a summation of conditions and pollutants and relies heavily on DO dynamics. The development and application of this index would require a detailed understanding of relationships between the normal dynamics of the component elements and how they respond to natural and anthropogenic stressors.
The republic of Ireland currently uses two indices for ecological health assessment of estuarine condition and health called, the Irish estuarine research programme indices (Wilson and Jeffrey, 1994). These consist of a pollution load index and a Biological quality index. The pollution load index combines concentrations of selected organic, nutrient and metal water quality variables, together with 'no effect' and 'threshold effect' levels for various estuarine biota. The concentrations of the candidate pollutants are compared to the no effect and threshold effect levels, given a weighting and combined.
For the biological quality index, estuary segments are classified into three possible classes: abiotic, opportunistic and normal, based on the abundance, and biomass of macrophytes and benthos. Considerable background information on the behavior of the candidate organisms in required during the development and application of this index.
The republic of South Africa currently uses an estuarine health index to define the ecological health of its estuaries (Cooper and Ramm, 1994). The estuarine health index is a linear combination of three separate indices including; a Biological health index, a Water quality index and an Aesthetic index. The biological health index is the number of fish species observed at a site, compared to number of species expected for the site, given its water quality and bar opening history. The water quality index is a linear combination of scaled ratings of DO, sediment oxygen demand, nutrient concentrations, E Coli and chlorophyll-a. The aesthetic index is a linear combination of ratings of floodplain landuse, riparian condition, estuarine odours, turbidity, weeds, incongruous buildings, oil sheen, bridges and noise.
The combined estuarine health index gave good discrimination of the health of South African estuaries and was consistent with other observations. Considerable background knowledge was required for selecting ratings of various elements of indices and for the application and interpretation of particular assessment ratings. The index suffers from the subjective nature of some of the assessments.
An index of disturbance has been developed for Tasmanian estuaries called the Stabilised disturbance index (Edgar et al., 1998). This index is the sum of proportional abundances of each benthic macrofaunal species observed, multiplied by the resulting coefficient from a Spearman correlation between human population and macrofaunal abundance. Stabilisation was achieved following 12 iterations where the initial human population correlation coefficient for each species, was replaced by the estimated disturbance index from each previous step.
The method requires spatially and temporally representative samples of macro-invertebrate abundance and human population data by catchment. The index produced good discrimination between estuaries based on disturbance. The stabilised disturbance index was better correlated to salinity than to human population in catchment. This index assumes that population of the catchment is proportional to disturbance. This may not be the case for broad-acre rural areas, throughout much of Australia where a relatively small population may have caused major changes in sediment and nutrient loss to receiving waterways. Catchment clearing may provide a better measure of anthropogenic stress in these situations. This index has potential regional significance for Tasmania, but would require additional work to test its application for other areas.
"A series of valid and objective protocols for sampling and analyzing biological communities is now beginning to emerge in the literature, but in many ways, the detailed research may seem a little esoteric to the people who are faced with putting these recommendations into practice and for whom the day to day business of environmental monitoring, is almost always a compromise between the scientific ideal and political, financial and logistical constraints. Quick and dirty techniques are usually called for .... (R.M. Warwick, 1993 pp 64)
Australian estuaries are highly dynamic systems and estuarine organisms by their very nature are eurytolerant (Wilson, 1994). This along with the various strategies that have evolved to accommodate the variability in conditions, present difficulties when attempting to use these organisms as indicators of anthropogenic impacts and change in estuaries. Estuarine species normally display a complex and dynamic pattern of maturity, disturbance and recolonisation (Poore and Rainer, 1974) in response to the normal cycles and patterns of salinity (John, 1987), currents, wave induced turbulence, dissolved oxygen, combined with episodic flooding (Moverley et al., 1986; Eyre and Twigg, 1996). Antecedent conditions may also have a profound influence on the nature of estuarine communities and their response to natural and anthropogenic stressors (Rainer, 1981).
Effective indicators of ecosystem health must be cost-effective, be based on the ecological health of biota and include an understanding of historical and current behavior. They should not apply only to a narrow geographical group of organisms in a limited area and should be sensitive enough to indicate anthropogenic stress while having limited sensitivity to natural variation (Tracy and Hough, 1995). Ecosystem restoration projects in the short term, and broad goals related to a self-maintaining ecosystem in the long term, will undoubtedly involve simultaneous monitoring at many levels of biological organization to provide a clear picture of ecosystem condition. Transferability of many community/ecosystem parameters across ecosystem boundaries facilitates their use as indicators that express changes at regional scales (Cairns Jr. et al., 1993).
Much has been written about the nature of indicators, their strengths and weaknesses and how they have been applied. There is little information however on the evaluation and selection of appropriate indicators although several frameworks for developing a suite of indicators for assessment, diagnosis and early warning have been presented (Cairns Jr. et al., 1993; Marshall et al., 1993; Rapport, 1995; Schaeffer, 1996). Judging the appropriateness of the chosen indicator suite is problematical. There is little published information on how to evaluate the suitability of selected indicators 'before the event', even though it is possible to assess the frequency of false positives and false negatives 'after the event'. All studies are selective with regard to which components and attributes of the biota should be investigated. The choice frequently depends on local expertise and research interests, rather than on an objective decision about what biological data are most appropriate for the problem at hand (Warwick, 1993; Whitton and Kelly, 1995).
Problems arise when attempting to derive biological early warning indicators of estuarine eutrophication. In most instances, by the time effects are observed using standard measures (hypolimnial hypoxia, reduction in diversity of benthic invertebrates or phytoplankton blooms), considerable damage has already occurred. Warwick (1993) has recognised the urgency for developing reliable indicators of ecosystem health in the face of widespread and ongoing environmental degradation, the reality of funding and political imperatives, the need to use best-bet indicators and to refine them and incorporate scientific rigor through time.
"......quick and dirty techniques are usually called for.What we need to know is how quick they can be made and just how dirty they really are!".... (R.M. Warwick, 1993 pp 64)
Clearly there needs to be iterative loops to refine and improve indicators, monitoring programmes, policy development and management over time. Estuaries by their very nature are however 'slow systems' with decadal time constants for the iterative refinement loops.
The following sections discuss the utility of various trophic groups as indicators of ecosystem health.
Autotrophic and heterotrophic protists perform key functions in energy flow and elemental cycling in freshwater, estuarine and marine food webs. Phytoplankton, together with benthic microalgae, are responsible for much of the primary production in estuarine habitats. This energy is transferred to higher trophic levels in the food chain mainly through the activity of protozoan and zooplankton grazers, which consume both algae and the bacterial assemblages that thrive off algal exudates. Phytoplankton also play an important role in the biochemical transformations of trace metals. Ecosystem-level response to toxicants may be set, in part, by the efficiency by which phytoplankton can act as agents of bioaccumulation and trophic transfer (Cloern, 1996). Thus the integrity and form of the entire ecosystem is closely linked to the functioning of these organisms (Cairns Jr. and McCormick, 1993).
Phytoplankton (and benthic microalgae) have been successfully used to describe current and historical nutrient enrichment, salinity and pH profiles (Kelly and Whitton, 1995; ten Cate et al., 1993) throughout the world. Diatom community structure has been used to assess the success of water quality improvement programs for the Cuyahoga River (USA). After 18 years species were found to have increased from 75 to 105 and there was a reduction in pollution tolerant species in favour of susceptible species (Brown and Olive, 1995). Improvements in diatom diversity were consistent with improvements in macro-invertebrate and fish diversities.
Although considerable progress has been made in using diatom indicator species to assess current and past water quality in freshwater lotic and lentic systems, little work has been done in marine and estuarine situations (Cairns Jr. and McCormick, 1993). There are currently major gaps in the Australian literature regarding the use of estuarine phytoplankton for assessing ecological health.
Chlorophyll variability has been widely used as an index of the phytoplankton population response to physical variability. Shifts from diatom-dominated communities to other phytoplankton groups such as chrysophytes, dinoflagellates and cyanophytes have accompanied increased N and P availability, relative to Si from anthropogenic sources.
Phytoplankton communities have been found to either increase, decrease or retain the same median biovolume (size strata) in response to anthropogenic stressors through shifts in dominance by various species and selective grazing by zooplankton. The loss of larger zooplankton after applications of pesticide to experimental lakes, was followed by dominance by large phytoplankton species. Microzooplankton were less affected by pesticide applications and maintained grazing control over smaller phytoplankton species (Havens and Hanazato, 1993). It is therefore probably not possible to use changes in the size strata of phytoplankton to describe ecological health.
The utility of phytoplankton as environmental indicators depends on the type of stressors. Because of the strong influence of hydrodynamic processes on their distribution, phytoplankton may be useful in integrating conditions across a larger area, but have limited utility for describing local stressors compared to benthic or attached forms, which may be useful locally, but have limited utility over a wider area (Cairns Jr. et al., 1993). Analysis of the remains of siliceous phytoplankton assemblages (diatoms, chrysophytes) in sediments, possibly provides the best available record of environmental conditions over the last 102 - 104 years (Dixit et al., 1992; Blinn, 1993; Smol, 1995a; Smol, 1995b).
Because of their nutritional needs and their position at the base of aquatic food webs, algal indicators provide relatively unique information concerning ecosystem condition compared with commonly used animal indicators (McCormick and Cairns, 1994; Patrick and Palavage, 1994). Phytoplankton, in particular, respond rapidly and predictably to a wide range of pollutants and thus provide potentially useful early warning signals of deteriorating conditions and the possible causes (Dixit et al., 1992; McCormick and Cairns, 1994; Whitton and Kelly, 1995).
One of the major impediments to using planktonic organisms for inferring the condition of estuarine health is the considerable vertical, horizontal and temporal heterogeneity displayed by these organisms in both disturbed and undisturbed systems (Scott, 1979). Community structure of phytoplankton communities has been used to describe conditions in estuaries, but spatial and temporal heterogeneity of chemical constituents, particularly salinity, confounds their use. Salinity gradients probably have a greater influence on community structure than the presence or absence of organic and nutrient pollution.
Eutrophication is an ecological process, so it should be studied from an ecosystem perspective that considers all the interacting physical, chemical, trophic and life history processes of phytoplankton population fluctuation. Phytoplankton communities and in particular diatoms, offer considerable potential for defining the effects of organic enrichment and eutrophication (Kelly and Whitton, 1995; ten Cate et al., 1993). Their role in trace metal and toxicant transfer to other estuarine biota should not be ignored. Particular groups of phytoplankton are potentially harmful to humans and they need to be screened on an ongoing basis. The need for baseline data, the requirement to standardize for spatial and temporal heterogeneity, particularly salinity and the required level taxonomic resolution will however add significant overheads to using phytoplankton as indicators of ecological health however.
Benthic microalgae may represent a higher biomass than that of phytoplankton in the overlying water column (Lukatelich and McComb, 1986a). They may act to reduce resuspension of sediments through the production of exudates and mats which bind sediments, are an important food source for deposit feeding benthos and play a significant role in benthic nutrient flux. Periphyton and epiphytic macroalgae have been found to strongly influence the amount of light reaching seagrasses and other benthic macrophyte communities and have thus been implicated in the loss of these keystone habitat components. Benthic microalgae may also contribute significantly to oxygen production and as such are an important component of the estuarine ecosystem.
Periphyton and benthic microalgae have been used extensively as water quality monitors in freshwater systems. Their small size, quick developmental response, physiological and taxonomic diversity and cosmopolitan distribution have made them an ideal community for evaluating the environmental quality of aquatic habitats, particularly as they lend themselves to experimental manipulations. There have been fewer investigations of this type into estuarine biota. There have been a number of Australian studies that have investigated aspects of benthic microalgal and periphyton colonisation, succession and production. Few of these investigations have defined the potential for these organisms to be used as indicators of ecological health and as such, there are major gaps in the local literature.
The abundance and diversity of benthic floral assemblages is influenced by season, salinity, substratum or sediment characteristics, tidal dynamics, competition, predation and nutrient availability. Light availability appears to have less impact on benthic microalgae than it does on macroalgae or seagrasses.
Following disturbance, colonization of substrata has been found to increase through immigration and reproduction; and decrease through emigration, mortality and grazing. The effect of water movement or sheer stress was found to influence the community structure of periphyton with different species having significantly different abilities to resist structural disturbance, but this ability can be modified by nutrient enrichment (Biggs, 1995).
Periphyton communities lend themselves to experimental manipulation through the use of artificial substrata. The colonization of substrata is influenced by the type of substratum being used. Artificial substrata offer certain advantages and disadvantages over natural substrata including:
Disadvantages of artificial substrata include:
Periphyton and benthic microalgae, being relatively sessile organisms, have potential to provide information on the ecological condition at the local scale with reduced ability to convey information for larger spatial scales. Benthic or attached periphytic communities may be superior to phytoplankton communities for assessing changes in local environmental conditions relating to trophic status (Cairns Jr. et al., 1993) because of their sedentary nature, ubiquity and short generation times.
Standardising for the effects of season, salinity, substratum or sediment characteristics, tidal dynamics, competition, predation and nutrient availability and overcoming the considerable spatial and temporal variability may require considerable additional investigations. Because of their importance as a food source for benthic deposit feeders and their role in oxygen production and nutrient flux, benthic microalgae may be important component of combined indicators that use several trophic groups.
Zooplankton play an important role in trophic transfer from primary producers to secondary consumers higher in the food web. Zooplankton may also play a significant role in settling of organic particles by ingestion and incorporation into fecal particles. This mechanism has been implicated in enhancing the removal of low solubility toxicants bound to organic and soil particles from the water column. Certain zooplankton species may also influence the evolution and species composition of plankton communities through selective grazing pressure.
Zooplankton have been extensively used to infer the condition of freshwater ecosystems. Daphnids and Cladocerans have formed the basis of many bioassay investigations into the impact of various toxicants in freshwater ecosystems. There was a shift in the community composition of zooplankton exposed to acidification and pesticides in freshwater systems. Larger species were replaced by smaller, tolerant, opportunistic species, but the effect was not consistent in all situations. Phytoplankton numbers increased to bloom proportions following the loss of zooplankton grazers (Havens and Hanazato, 1993). There have been few published accounts of the suitability of zooplankton as indicators of ecosystem function or health in Australian estuaries.
Zooplankton abundance, productivity and fecundity are influenced by seasonal factors including temperature and salinity (Dias, 1994; Hoffmeyer, 1994; Turner, 1994), the nature and abundance of food organisms (Dam et al., 1994; Harris, 1994) and the nature of predation (Kimmerer and McKinnon, 1985).
Zooplankton productivity is generally tightly coupled to phytoplankton productivity (Mallin, 1991; Mallin and Paerl, 1994; Carter et al., 1995), but this relationship has been found to be influenced by secondary predation and in extreme cases, by the presence of cyanophyte blooms (Mallin, 1991; Harris, 1994; Mallin and Paerl, 1994; Carter et al., 1995). Investigations of sex ratios, egg numbers and body size in zooplankton communities in the Brisbane River estuary, found that females were usually more abundant than males in the less abundant species, but that the sex ratio was nearer 1.0 for common species (Bayly, 1965).
There was considerable variation in zooplankton standing crop, both spatially and temporally within and among four Victorian estuaries (Neale and Bayly, 1974), but little change in temporal and spatial distribution in the Derwent estuary, where there was only moderate displacement of the salinity field under high and low freshwater flows (Taw and Ritz, 1978). The presence of marine zooplankton indicator species has been used to infer intrusion of marine waters into estuaries (Taw and Ritz, 1978; Taw and Ritz, 1979).
Zooplankton have a limited ability to add to the paleolimnological record (Leavitt et al., 1994) because of their relatively soft bodies, although in some situations chitinous remains of zooplankton are present. Metabolic by-products, a suite of alkenes, sterols alcohols and a range of pigments have however, been used as biomarkers of zooplankton activity (Shuman and Lorenzen, 1975; Yunker et al., 1995).
Zooplankton exhibit considerable spatial and temporal variability in their distribution. In addition to normal tidal dispersal, many species of zooplankton undergo diel vertical migration. As well as avoiding visual predators, diel vertical migration may assist zooplankton maintain their preferred horizontal distribution in tidal estuaries. The lunar cycle may also influence diel vertical migration patterns and thus horizontal distributions (Jerling and Wooldridge, 1992). Zooplankton distribution may thus be patchy and highly variable, with spatial separation of classes and differential age- and sex-specific responses to hydrodynamic processes.
In tidal estuaries, pulses in active vertical migration and passive tidal dispersal mean that multi-level sampling on both ebb- and flood-tides is a prerequisite for reliable density estimation of estuarine zooplankton (Schlacher and Wooldridge, 1995). The considerable spatial and temporal variability of zooplankton communities means that zooplankton appear to offer only limited scope as environmental indicators but may be useful as elements of biotic indices across trophic groups.
Despite potential difficulties, zooplankton communities need to be screened as part of a comprehensive evaluation of candidate health indicators, because of their potential influence on phytoplankton and periphyton abundance and diversity and their roles in trophic energy transfer and toxicant cycling.
Benthic macro-invertebrates have been most used as indicators of eutrophication, organic enrichment, thermal pollution and toxicants. In particular, where large organic loads have occurred, there appears to be loss of larger species in favour of smaller opportunistic species (Warwick, 1986). This may also result in reductions in biomass and diversity where the impacts have been severe (Forbes and Lopez, 1990; Nielsen and Jernakoff, 1996). Where impacts are less pronounced, there may be an increase in diversity as both the original fauna together with opportunist species are observed, consistent with the intermediate disturbance hypothesis (Connell, 1978). Many observed shifts in community structure have been attributed to the differential ability of component species to tolerate decreases in hypolimnetic dissolved oxygen concentrations following eutrophication (Cairns Jr. et al., 1993).
Species richness of benthic macrofauna may be reduced and productivity may be either reduced or increased adjacent to thermal plumes depending on; ambient conditions, the nature of the benthos and on the severity of the thermal pollution (Kennish, 1991). The response of soft-sediment fauna to metal accumulations is not consistent. For example, artificially enhanced copper concentrations in sediments produced inconsistent results across taxa (Morrisey et al., 1996). Crustaceans, bivalve molluscs and larval polychaetes, have been found to be more susceptible to Zn than adult polychaetes, gastropod molluscs and fish (Mance, 1987). Benthic macrofauna may also play an important role in the cycling of bio-accumulative toxicants to higher trophic groups through bioturbation of the sediments and capture by higher order prey organisms. The nature of bioaccumulation and trophic transfer processes for benthic macrofauna, may influence ecosystem-level impacts of toxic materials (Cloern, 1996).
Benthic macroinvertebrates are probably the most studied trophic group in Australian estuaries (see counts by group Appendix 2). There have been numerous investigations into relationships between abundance and diversity, following organic enrichment, eutrophication and contact with thermal plumes. There have been few investigations into the impact of chronic low-level or acute inputs of toxicants, apart for some isolated hot spots. The impact of toxicants on benthic macrofauna was to reduce abundance, diversity and size strata, but the effect was not consistent in all cases.
The advantages of soft-bottom macrobenthos for describing aspects of estuarine health are that they are relatively non-mobile and are therefore useful for studying the local effects of pollutants; their taxonomy is relatively easy; and it may be possible to detect the impacts of anthropogenic disturbance at coarser taxonomic resolution than species; quantitative sampling is relatively easy; there is an extensive international literature on the nature of impacts of pollutants on macrobenthic communities (Warwick, 1993).
Because the net effect of increased nutrient, sediment or organic loadings to estuaries may be a shift to smaller opportunistic deposit-feeding macrobenthos, changes in the size strata, abundance and diversity of benthic macrofaunal communities, may be reliable indicators of disturbance. Physico-chemical measures should also be evaluated to define their influence on benthic community structure (Wright et al., 1984; Growns et al., 1992), but unless account is taken of recent past history, the results may appear to be ambiguous.
Potential disadvantages likely to accrue from using macrobenthos include: the requirement to process sediments to extract animals either fresh or after preservation; the potential response of macrobenthic communities to a pollution event may be slow, and because recolonization dynamics are complex, may have strong seasonality (Saenger et al., 1980; Underwood and Anderson, 1994); community dynamics are not well understood in all situations and; there are complex patch dynamics and multiple stable endpoints in communities not subject to anthropogenic disturbance (Poore and Kudenov, 1978b; Underwood and Anderson, 1994).
It has also been concluded that disturbance-induced reductions in the size strata, abundance or diversity of macrobenthos may only occur after considerable environmental damage has occurred and these types of indicators offer little early-warning or diagnostic precision.
The structure of benthic macro-invertebrate communities, when combined with an assessment of substrate type, environmental variables, settlement dynamics may be used as powerful indicators of anthropogenic stress. Standardising the spatially and temporally variable distributions of benthic macrofauna and the nature of interactions with other trophic groups, is problematical, but when calibrated, these types of indicators may provide a good assessment of the ecological condition of estuaries.
There are a number of recently published Australian reports recommending various environmental indicators for SOE reporting and for use in assessing the ecological health of ecosystems (OECD, 1994; ANZECC, 1998; Fairweather and Napier, 1998; Ward et al., 1998a; Ward et al., 1998b). Estuarine managers require a rigorous evaluation of the statistical power and application of these indicators to underpin the difficult task of assessing the early onset of adverse changes in the ecological health of Australian estuaries (Deeley, 1999).
| Table A-1.1 Pressure indicators used globally to assess aspects of estuaries (includes USEPA stressor indicator category). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application CDRa | Cost | Ref |
| Catchment | |||||||
| Population density | Population and demographics | Catchment Long term | Population by location | Broad indication of pressures Locale specific | Core, in use | low | 12 7 |
| Catchment landuses and management practices | Catchment landuse mix and management practices used by land managers | Catchment Long term | Land use maps, summary statistics, audit of management practices against codes of best practice | Coarse indication of potential loss rates Delayed links between manage-ment and runoff quality | Core, in use, potential to link to policy response indicators | Moderate | 10 12 |
| Catchment, riparian vegetation status | Nature of catchment vegetation, condition of riparian vegetation | Catchment long term | Catchment vegetation maps, riparian condition assessments | Remote sensing for large areas Need links with runoff quality | Core, in use, potential to link to response indicators | Low | 10 |
| Soil erosion | Assessment of soil loss | Catchment, stream segments Longterm, & instantaneous | Streamline condition assessment, relative changes | Indication of sediment delivery to receiving waterways Poor links to impacts on biota | Core, in use, potential to link toresponse indicators | Low | 10 |
| Runoff patterns | Flood frequency, peak flow velocities | Catchment, stream segments Short term | Continuous discharge measurements, time series analysis | Potential for pollutant transport, success of detention structures Poor links to downstream impacts | Core, in use | Low | 9 10 |
| Catchment drainage density | Length of streams and artificial drains per unit area of catchment | Catchment Long term | Stream, artificial drain length, catchment area | Good for estimating potential runoff from catchments Site specific | Developmental | Low | 2 |
| Stream width to depth ratio, pool-riffle sequences | Disturbance to stream channel, habitat diversity | Stream segments Long term | Stream channel condition assessment | Habitat diversity, potential for flooding, erosion Need baseline and comparisons | Developmental, potential to link to policy indicators | Low | 16 |
| Runoff quality, nutrients, organic matter, sediment | Concentrations or mass loads of nutrients, sediments | Catchment, stream segments Longterm, & instantaneous | Statistically representative samples across season and flow strata for concentrations For mass loads, flow, nuts, robust interpolation methods | Indicator of mass flux to receiving waters, changes in water quality, hot spot identification Impact on receiving environment uncertain | Core, in use, links to policy response indicators | Low - moderate | 13 |
| Toxicant inputs | Concentrations of heavy metals, synthetic organics, petroleum products in runoff, industrial discharges, spillages | Catchment, stream segments Instantaneous Longterm trends, | Statistically representative samples across season and flow strata by constituent | Identification of pollutant hot spots, success of pollution control Many compoundsto screen, most low water solubility, adsorbed to particles | Core, in use, potential to link to policy response indicators | Moderate to high | 9 19 |
| Note: For key to references, see Table A-1.5. CDRa = Core indicators - in use, Developmental indicators - links established, methods required, Research Indicators - links required. | |||||||
| Table A-1.1 ctd Pressure indicators used globally to assess aspects of estuaries (includes USEPA stressor indicator category). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application CDR | Cost | Ref |
| Estuary | |||||||
| Toxicant concentrations in estuarine waters | Concentrations ofheavy metals, synthetic organics, petroleum products in estuarine waters | Estuary, or segments Short term | Statistically representative samples spatially and temporally, nature of active compounds | Links to potential impacts on biota Most, low water solubility, need ultratrace analytical methods | Core, in use, potential to link to policy response indicators | Moderate to high | 19 |
| Toxicant concentrations in sediments | Concentrations of heavy metals, synthetic organics, petroleum productsin estuarine sediments | Segments, estuary Short to medium term | Statistically representative samples spatially, nature of active compounds | Links to potential impacts on biota Bound to sediments, trace analytical methods | Core, in use, potential to link to policy response indicators | Moderate | 12 17 19 |
| Sediment toxicity | Bioassay using biota and contaminated sediments | Sites, Segments Instantaneous | Organism response, exposure can be experimentally manipulated | Direct measure of toxicity and impacts on biota Test organism specific | Research | Moderate to high | 17 |
| Note: For key to references, see Table A-1.5. | |||||||
| Table A-1.2 Abiotic Condition indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application, CDR | Cost | Ref |
| System | |||||||
| Particle retention efficiency | Ratio of estuarine volume to annual freshwater inflow | Estuary Long term | Annual streamflow, estuarine mean volume | Broad assessment of capacity to trap particles, adsorbed pollutants Assumes constant sediment trapping, less meaningful for estuaries with flow seasonality | Core, in use USA | Low | 16 |
| Dissolved concentration potential | Ratio of flushing to dissolved pollutant inflow | Estuary Long term | Average annual freshwater fraction by segment, dissolved pollutant inflow | Broad assessment only, probably matches the large errors typically for estimates of dissolved pollutant flux Assumes vertical homogeneity, less meaningful for estuaries with major seasonal flow variability | Core, in use in USA | Low-moderate | 16 |
| Flushing (retention time) | Estimate of retention time using conservative mixing plots | Lower estuary Long term | Salinity profiles | Simple measure of residence time Links to dynamics of other constituents poor | Core. in use | Low | 8 |
| Tidal prism, water levels | Tidal prism in estuary | To limit of tidal influence Long term | Tidal range through estuary | Simple calculation, measure of flushing, tidal attenuation Links to dynamics of other constituents poor | Core. in use | Low | 8 |
| Capacity for stratification | Degree of mixing and potential for stratification | Segments Short term, &instantaneous | Salinity, temperature, DO, profiles for at risk periods, bathymetry | Indication of susceptibility of estuary Links to dynamics of other constituents, biota response, poor | Core, in use | Low | 12 |
Note:For key to references, see Table A-1.5. | |||||||
| Table A-1.2 ctd Abiotic Condition indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential applicationCDR | Costs | Ref |
| Water quality | |||||||
| Salinity | Distribution of salt water in estuary, stratification | Estuary, segments Instantaneous & long term patterns | Vertical, horizontal salinity profiles, spatial and temporal assessments | Understanding of habitat condit-ions, seasonal dynamics, potential for stratification, dilution | Core, in use | Low | 12 |
| Temperature, thermal plumes | Distribution of temperature in estuary, stratification | Estuary, segments Instantaneous & long term patterns | Vertical, horizontal temperature profiles, spatial and temporal assessments | Understanding of habitat conditions, seasonal dynamics, potential for stratification, threats | Core, in use | Low | 12 |
| pH | Distribution of pH in estuary | Estuary, segments Instantaneous & long term patterns | Vertical, horizontal pH profiles, spatial and temporal assessments | Low values show freshwater or acidic industrial inputs High values indicate phytoplankton blooms or alkaline industrial inputs | Core, in use | Low | 12 |
| Clarity, turbidity (Secchi depth) | Transparency of water in estuary | Estuary, segments Instantaneous& long term patterns | Secchi depth, or light attenuation profiles | Indication of solids inputs and resuspension, phytoplankton, light available for photosynthesis in benthic communities High attenuation in natural tannin-rich waters | Core, in use, potential to link to policy response indicators | Low | 14 |
| Dissolved oxygen | Dissolved oxygen concentrations throughout estuary | Whole estuary, segments, bottom waters Instantaneous & long term patterns | Vertical, horizontal profiles with spatial and temporal assessments, diurnal patterns, or continuous measurement Instantaneous measures direct measure of stress of benthic communities | Hypolimnetic supersaturation shows phytoplankton blooms, benthic hypoxia and anoxia directly related to stress in fauna Highly dynamic measure, need larger number of readings to define changes in estuarine condition | Core, in use, potential to link into policy response indicators | Low | 14 |
| Nutrient concentrations in water, N:P:Si ratios | Concentrations and chemical forms of nutrients in estuarine waters | Estuary, or segments Instantaneous & long term patterns | Statistically representative samples spatially and temporally, bottom and surface waters | Enrichment status, potential limiting nutrients. Mass balance approach, effective in management of eutrophication. Causal links difficult to establish, need large number of samples to determine change in conditions | Core, in use, potential to link to policy response indicators | Moderate | 14 |
| chlorophyll-a Phaeophytin | Distribution of plant pigments in estuarine waters | Estuary, or segments Instantaneous & long term patterns | Statistically representative samples spatially and temporally integrated samples | Measure phytoplankton activity Dynamic measure, need large number of samples to determine change in condition over time | Core, in use, potential to link to policy response indicators | Moderate | 13 |
| Note:For key to references, see Table A-1.5. | |||||||
| Table A-1.2 ctd Abiotic Condition indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application, CDR | Costs | Ref |
| Sediments | |||||||
| Particle size distribution | Distribution of sediment types, benthic habitats | Estuary, or segments Long term | Samples from surface and or deeper layers | Provides baseline information and habitat description, indication of genesis and sedimentary processes | Core, in use | Low | 12 |
| Organic matter content | Distribution and organic content of sediments | Estuary, or segments Long term | Samples from surface and or deeper layers | Provides baseline information and habitat descriptionVery high values may indicate organic pollutant loadings | Core, in use | Low | 12 |
| Sediment oxygen demand (SOD), Redox discontinuity | Respiratory demand of sediments Oxygen flux to sediments | Estuary, or segments Instantaneous& medium term | Undisturbed cores Redox discontinuity depth | Measure of oxygen state and demand of sediments.Risk of hypoxia and anoxia Links to biota not clear, site specific, heterogeneous results | Development | Moderate | 6 12 |
| Sediment nutrient concentrations | Forms and concentrations of nutrients in sediments | Estuary, or segments Short term | Samples from surface and or deeper layers | Indicates partitioning of nutrients and degree of enrichment Links to regeneration mechanisms unclear | Core, in use, may be linked to policy response indicators | Moderate | 12 |
| Note: For key to references, see Table A-1.5. | |||||||
| Table A-1.3 Biotic Condition indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential applicationCDR | Costs | Ref |
| Community | |||||||
| Abundance | Counts of specific organisms or groups of organisms, includes phytoplankton cell counts, or cover in colonials | Estuary, segments, sites Short term | Representative samples, spatially and temporally to define dynamics and distribution of populations | Simple method for describing biota, little taxonomic expertise required Dynamic, links to processes and interactions with diversity unclear | Core, in use | Low to moderate | 12 |
| Biomass | Weights of specific organisms or groups of organisms | Estuary, segments, sites Short term | Representative samples, spatially and temporally to define dynamics of populations | Simple method for describing biota, little taxonomic expertise required Links to processes and interactions with diversity unclear | Core, in use | Low to moderate | 12 |
| Species richness | Counts of species for various trophic groups standardised by abundance | Estuary, segments, sites Short to medium term | Representative samples, spatially and temporally to define dynamics of populations | Method for establishing diversity of communities, disturbance Taxonomic expertise, baseline required | Core, in use | Moderate | 3 |
| Diversity | Indices of diversity calculated from abundance of species | Estuary, segments, sites Short to medium term | Representative samples, spatially and temporally to define dynamics of populations | Indication of disturbance High taxonomic expertise required links to anthropogenic disturbance not always clear | Core, in use | Moderate | 3 |
| Productivity | Rate of metabolic processes in selected organisms or groups of organisms | Estuary, segments, sites Instantaneous & short term | Healthy samples of organisms of interest, maintenance of organism for duration of observations, measurement of metabolic processes of interest | Metabolic processes provide information on biotic responses to conditions, lends to experimental manipulation Unknown applicability of results to field situations | Development, research | Moderate to high | 14 |
| Size strata | Measurement of sizes of organisms | Estuary, segments, sites Short to medium term | Direct measurements of size or calculated from numbers and biomass | Indication or degree of disturbance in community Require comparative results for interpretation | Development, phytoplankton may increase in size with nutrient enrichment | Moderate | 3 |
| Potentially harmful phytoplankton or byproducts | Presence of cyanophytes (blue-green)dinophytes(red tides) or other harmful phytoplankton (e.g. DSP, PSP) | Estuary, segments, sites Instantaneous | Samples of phytoplankton, identification, risk assessment, toxin assessment by HPLC, microtox, mouse bioassay, shellfish tissues for screening | Screening for potential human, animal or aquaculture health threats Toxin assessment difficult, dynamic nature of blooms, DSP, NSP toxins and organisms cryptic | Core, in use | Moderate | 3 |
| Periphyton accumulation Autotrophic index | Periphyton colonisation, growth Ratio of heterotrophs to autrotrophs, indication of organic loadings, nutrient supply | Estuary, segments, sites Instantaneous & medium term | Samples of periphyton in situ or from artificial substrata | Indication of nutrient and dissolved or particulate organic matter status Site specific, applicability of results from artificial substrata | Research for estuaries (in use for freshwater streams in USA) | Moderate | 1 |
| Seagrass cover, depth of growth limits, epiphyte loads Macroalgae | Density, distribution of seagrasses, macroalgae, epiphytes | Estuary, segments, sites Medium term | Macrophyte assessment methods. Remote sensing, air photos, spot dives, fixed transects, harvested samples | Habitat health, Integration of ambient estuarine conditions, describe changes in nutrient loads or turbidity Links to causal mechanisms not clear | Core, in use | Moderate | 18 |
| Benthic community indices | Ratios of functional groups of benthos | Estuary, segments, sites Medium term | Spatially and temporally representative samples of benthos | When calibrated for particular location offer considerable diagnostic precision Site specific, need understanding of baseline conditions | Core, in use in USA, Research for Australian estuaries | Moderate to high | 12 |
| Indicator species | Presence, counts of indicator species | Whole of estuary, segments | Spatially and temporally representative samples of indicator species, autecology | Indicator of particular conditions Detailed autecology of indicator organisms required, baselines | Research | Moderate | 3 |
| Fishery catch per unit effort | Effort required to harvest seafood units | Estuary, segments, sites Medium term | Fishing effort, equipment used, catch, spatial and temporal trends | Can detect changes in populations of fisheries Links between sustainability and early warning not clear, may only signal decline of community | Core, in use, research and development for some species | Low to high depending on data capture | 12 |
| Introduced species | Assessment of pest species | Estuary, segments, sites Medium term | Counts and identification of introduced exotics | Early warning for invaders if surveillance robust Require knowledge of prior baseline populations | Core, in use, Development for some species | Moderate | 3 |
| Note: For key to references, see Table A-1.5. | |||||||
| Table A-1.3 ctd Biotic Condition indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application, CDR | Costs | Ref |
| Organism level indicators | |||||||
| Biomarkers | Biochemical processes responding to stressors e.g. mixed-function oxidase, metallothioneins | Organisms, Instantaneous | Representative samples of organisms of interest, baseline conditions, exposure response, Lends to experimental manipulation | Direct measure of stress in organisms Genetic variability in response of community members, need for baseline information | Research | Moderate to high | 11 |
| Developmental stability analysis | Presence of 'abnormal' organisms, attributes | Organisms, communities Short term | Representative samples of organisms of interest, baseline conditions, exposure response | Indirect measure of stress in organisms Genetic variability in response need for baseline information, irreversaibility from past exposure | Research,Use for TBT and imposex in WA, binary response, links to rates of exposure unclear | Moderate | 4 6 |
| Disease, incidence | Disease occurrences in organisms, communities | Organisms, communities Short term | Representative samples of organisms of interest, baseline conditions, exposure response | Indirect measure of stress in organisms Genetic variability in response of community members, need for baseline information, causal links unclear | Research | Moderate | 3 |
| Note:For key to references, see Table A-1.5. | |||||||
| Table A-1.4 Combined Indicators used globally to assess aspects of estuaries (includes OECD State indicators, USEPA Habitat, Response and Research indicator categories). | |||||||
|---|---|---|---|---|---|---|---|
| Indicator | Description | Scales Spatial Temporal | Data requirements | Advantages / disadvantages | Potential application, CDR | Costs | Ref |
| Combined indicators | |||||||
| United Kingdom NWC | United Kingdom National Water Council index, combines DO status, biological quality of fish habitat and benthos with observable pollution | Estuary, segments Short term condition, long term trends | DO data, assessment of migratory fish passage and resident fish diversity and benthos, toxic or tainting substances, pollutant inputs and nature of detectable impacts | Summation of conditions and pollutants Relies heavily on DO dynamics, required considerable expert understanding for ratings and causal interpretations | Core, in use in UK | Low to moderate | 20 |
| Ireland ERP | Irish estuarine research programme indices. PLI -pollution load index, BQI -Biological quality index | Estuary, segments Short term condition, long term trends | PLI concentrations of selected organic, nutrient and metal wq variables, no effect and threshold levels BQI estuary segments classified to three classes: abiotic, opport-unistic and normal, based on abundance, and biomass of macrophytes and benthos | Assessment of condition of estuaries and change over time Need for representative samples of water quality and biota, considerable background information to establish ratings | Core, in use in Ireland | Low to moderate | 20 |
| South Africa EHI | South African estuarine health index = Sum of results from Biological health index, Water quality index and Aesthetic index. | Estuary, segments Short term condition, long term trends | Biological health index = number of fish species observed compared to number of species expected for the site. Water quality index = linear combinations of scaled ratings DO, SOD, nutrients, E Coli, Chlor-a Aesthetic index = linear combination of ratings of floodplain landuse, riparian condition, estuarine odours, turbidity, weeds, ugly buildings, oil sheen, bridges, noise. | Combined estuarine health index gave good discrimination of South African estuaries. Considerable background knowledge required for selecting ratings of various elements of indices. Subjective nature of some assessments | Core, in use in South Africa | Low to moderate | 5 |
| Tasmania Stabilised disturbance index DI | DI = Sum of proportional abundance of each benthic macrofaunal species multiplied by correlation between human population and abundance Stabilisation achieved from 12 iterations replacing initial human population correlation coefficient by species with DI derived from each previous step | Estuary, segments Short term condition, long term trends | Spatially and temporally representative samples of macro-invertebrate abundance, human population data by catchment | Produced good discrimination between estuaries based on disturbance.Stabilised DI more correlated to salinity than to human population in catchment Assumes that population of catchment proportional to disturbance, may not be the case for broad acre rural areas.Regional significance, requires evaluation for other regions | Research, commenced for Tasmanian estuaries | Moderate | 7 |
| Florida Panhandle estuary condition | Rating applied to DO, Benthos, Fish, Sediment state, Light, Debris | Estuary, region | Data on DO concentrations, light attenuation, sediment toxicant concentrations, diversity, abundance of benthos, fish, debris.A coloured scale from green for good to red for bad is used, individual ratings are represented on the coloured scale. | Information for indivdual variables is not lost through combining.Factors contributing to low or high ratings are obvious.Very good for information extension to the community. | Core, in use in USA | Moderate | |
| Note:For key to references, see Table A-1.5. | |||||||
Table A-1.5 Bibliography of references described in Tables A-1.1, A-1.2, A-1.3 and A-1.4.
Number Source
1. APHA (1992) Standard methods for the examination of water and wastewater.American Public Health Association, Washington, D.C.
2. Bott, G.M. (1992) Environmental and landuse factors affecting phosphorus losses from estuary catchments of south west Western Australia. In "Fertilizers and Eutrophication in South Western Australia".
3. Cairns Jr., J., McCormick, P.V. and Niederlehner, B.R. (1993)A proposed framework for developing indicators of ecosystem health [Review]. Hydrobiologia263, 1-44.
4. Clarke, G.M. (1994)Developmental stability analysis:An early-warning system for biological monitoring of water quality. Australian Biologist7, 94-104.
5. Cooper, J.A.G. and Ramm, A.E.L. (1994)The estuarine health index: A new approach to scientific information transfer. Ocean Coastal Management25, 103-41.
6. DEP (1996)Southern metropolitan waters study (1991-1994). , Department of environmental protection of Western Australia.
7. Edgar, G.J., Barrett, N.S. and Graddon, D.J. (1998)A classification of Tasmanian estuaries and Assessment of their conservation significance: an analysis using ecological and physical attributes, population and landuse. , Parks and Wildlife Service, Department of Environment and Land Management, Hobart, Tasmania.
8. Eyre, B. (1995)A first-order nutrient budget for the tropical Moresby estuary and catchment, north Queensland, Australia. Journal of Coastal Research11, 717-32.
9. Fairweather, P.G. and Napier, G.M. (1998)Environmental indicators for national state of the environment reporting - Inland waters. , Department of the environment, Canberra.
10. Hamblin, A. (1998)Environmental indicators for national state of the environment reporting - The land. , Department of the Environment, Canberra.
11. Holdway, D.A., Brennan, S.E. and Ahokas, J.T. (1995)Short review of selected fish biomarkers of xenobiotic exposure with an example using fish hepatic mixed-function oxidase [Review]. Australian Journal of Ecology20, 34-44.
12. Holland, A.F., (ed). (1990)Near coastal program plan for 1990: Estuaries. , U.S. Environmental Protection Agency, Environmental Research Laboratory, Office of Research and Development, Narragansett, RI.
13. Kinhill (1988)Peel-Harvey Environmental Review and Management ProgramStage II. , Kinhill Engineers Pty Ltd.
14. McErlean, A.J. (1981) Indicators and indices of estuarine overenrichment. In "Estuaries and nutrients" (B. J. Neilsen and L. E. Cronin, eds.), Humana Press, Clifton, New Jersey.
15. NOAA (1989)Strategic assessment and status of Gulf of Mexico estuaries to nutrient discharges. , National Oceanic and Atmospheric Administration.
16. Riding, T. (1992)NSW State rivers and estuaries policy:State of the rivers and estuaries - Environmental indicators - A literature review. , NSW Department of Water Resources.
17. Summers, J.K., Macauley, J.M., Engle, V.D., Brooks, G.T., Heitmuller, P.T. and Adams, A.M. (1993)Louisianian Province demonstration report: EMAP - Estuaries - 1991., U.S. Environmental Protection Agency, Office of Research and Development, Environmental Research Laboratory, Gulf Breeze, Fl.
18. Walker, D.I. and McComb, A.J. (1992)Seagrass degradation in Australian coastal waters. Marine Pollution Bulletin25, 191-5.
19. Ward, T.J., Butler, E. and Hill, B. (1998)Environmental indicators for national state of the environment reporting - Estuaries and the sea. , Department of the Environment, Canberra.
20. Wilson, J.G. (1994)The role of bioindicators in estuarine management. Estuaries17, 94-101.
Note: The references listed above are examples of the application of indicators, and are not intended to be a comprehensive listing, or to be the primary reference.
| Table A-2.1 Biological Inventory indicators used to assess the ecological health of plankton and benthic communities in Australian estuaries. | ||||||
|---|---|---|---|---|---|---|
| Indicator | Phytoplankton | Macroalgae | Seagrass | Periphyton /Micro-phytobenthos | Zooplankton | Benthic macro-invertebrates |
| Abundance / pigment concentrations / cell counts | 1-6 (6) High spatial and temporal hetero-geneity.Distinct seasonal assemblages, cyclical patterns of succession, valuable as indicators. | 7-9 (3) Seasonal cycles in growth and biomass accumulation. | 10,11 (2) Biomass negatively related to epiphyte biomass. | 12-15 (4) Significant proportion of primary production in estuary. Light of less importance for survi-val than for benthic macrophytes. | 16-21 (6) High spatial and temporal heterogeneity in distributions, diel vertical migration. Role in settling of particles with adsorbed pollutants as feacal pellets. | 22-42 (20) Abundance increases with high organic loading, impact less obvious with distance. Enriched communities high seasonality.Positive relationships with DO |
| Biomass | 3-6 (4) Increase in biomass with increased nutrients.N more potential limit than P, self-shading in blooms. | 43, 44 (2) Biomass influenced by light levels and nutrient availability. | 45 (1) Biomass and shoot density strongly related to light levels. | 15 (1) Biomass and produc-tion influenced by irradience, depth temperature, sediment type and nutrients. | 38-40 (3) Biomass reduction with with nutrient over-enrichment, increase with moderate enrich-ment. | |
| Cover | 7, 46 (2) Reduced cover with nutrient enrichment through light reduction | 35 (1) Cover of colonial suspension feeders reduced through increased turbidity, sediment. | ||||
| Nutritional status | 48 (1) Rapid increase in opportunistic species with nutrient enrich-ment. Relationships between density and N, tissues good indicator of nutrients. | 44 (1) Strong negative relat-ionships between N and biomass, uptake.Tope of macroalgal blankets active, lower portion low DOnutrientregeneration | 49 (1) No clear relationships between growth and nutrients. Porewater P may limit in carbonate sediments, N may limit in terrigenous sediments | 15 (1) Relationships with N and P loading and biomass or production not clear. Reduced NH4 efflux from sediments under illumination. | 17, 19 (2) Pelagic nutrient regeneration through grazing and release of feacal pellets.Positive relationship between nutrient enrichment and biomass indirect through increased food. | 35-40 (6) Bioturbation by suspension feeders promotes nutrient regeneration.Relationships with biomass, nutrients unclear response from increased organic loading. |
| Size strata | 74 (1) Nutrient enrichment may result in larger species, oppostie effect to benthos. | 17, 53 (2) Some species may delay growth to larger size where visual predation high | 50 (1) Reduction in size strata with increased organic loading, smaller opportunists, loss of larger species | |||
| Note: Numbers in cells represent references, number in brackets shows count of references for topic.For key to references, see Table A-2.4. | ||||||
| Table A-2.2 Biological indicators of Community Structure used to assess the ecological health ofplankton and benthic communities in Australian estuaries. | ||||||
|---|---|---|---|---|---|---|
| Indicator | Phytoplankton | Macroalgae | Seagrass | Periphyton / Micro-phytobenthos | Zooplankton | Benthic macro-invertebrates |
| Species / taxon richness | 6, 68 (1) Reduction in diversity with nutrient enrich-ment, bloom species dominant. | 51 (1) | 15 (1) Positive response to nutrient enrichment, diatoms respond to nutrients may be preferentially grazed | 16, 52-54 (4) Zooplankton grazing may influence phytoplankton or zooplankton com-munity structure | 23, 25-28, 30-32, 34-40, 42, 47, 50, 55-62 (25) Aggregation to higher taxa shows impacts of mild and severe stress. | |
| Species diversity / Evenness | 63 (1) Major seasonal cycles in diversity, abund-ance in temperate estuaries, less varia-bility in tropical estuaries. | 51 (1) Opportunist species rapidly replace zoned perennial species as nutrient enrichment progresses | 19 (1) Significant differences in community and diversity on tidal cycles | 25, 35-40, 42, 47 (9) Reduction in diversity with severe stress organics.Mild dis-turbance increased diversity. | ||
| Community groupings / guilds | 68 (1) Increase in cyano-phytes, dinophytes with increased nutrient supply, use as indicators. | 27-28 (2) Deposit feeders may exclude suspension feeders through increasing turbidity.Suspension feeders may exclude deposit feeders through tubes consolidating sediments.Deposit feeders increase, suspension feeders decrease with increased organic loading, turbidity.Polychaetes higher tolerance to hypoxia. | ||||
| Note: Numbers in cells represent references, number in brackets shows count of references for topic.For key to references, see Table A-2.4. | ||||||
| Table A-2.3 Biological indicators of Community Function and Organism Response used to assess the ecological health of plankton and benthic communities in Australian estuaries. | ||||||
|---|---|---|---|---|---|---|
| Indicator | Phytoplankton | Macroalgae | Seagrass | Periphyton / Micro-phytobenthos | Zooplankton | Benthic macro-invertebrates |
| Colonization rates | 41-64,(2) Early colonists modify microlayer provide conditions for later colonisers | 41, 42 (2) Seasonality in colon-isation, successional sequence related to viability of repro-ductive adults releasing young. | ||||
| Productivity | 73 (1) Seasonal differences productivity not tem-perature effects, may be physiological pre-conditioning | 65, 73 (2) Productivity strongly related to light | 50 (1) Productivity includes food consumption, egg production, meta-bolism, growth | |||
| Egg production | 23 (1) Egg production of epifauna positively related to food avail-ability, negatively related to abundance. | |||||
| Indicator species | 2, 66-68 (4) Presence of harmful phytoplankton species good indicator | 20, 21 (1) Some species narrow salinity tolerance indicatorsof marine or fresh influences | ||||
| Biomarkers | 70 (1) Organic sterols produced by phyto-plankton and other plants good bio-markers | 69 (1) DNA damage in epi-fauna related to level of anthropogenic stress. | ||||
| Development stability analysis | 71 (1) Potential indicators of stress for zooplankton, antennae deformaties, egg production, respir-ation need calibration. | 71, 72 (2) Imposex in gastropodsrelated to proximity to TBT, past exposure irreversible. | ||||
| Note: Numbers in cells represent references, number in brackets shows count of references for topic.For key to references, see Table A-2.4. | ||||||
| Table A-2.4 Bibliography of references described in Tables A-2.1, A-2.2 and A-2.3. | |
|---|---|
| No. | Reference |
| 1 | Geddes, M. C. (1984) Australian Journal of Marine and Freshwater Research 35, 399-415. |
| 2. | Lukatelich, R. J. & McComb, A. J. (1986) Journal of Plankton Research 8, 597-618. |
| 3. | Revelante, N. & Gilmartin, M. (1978) Australian Journal of Marine and Freshwater Research 29, 9-18. |
| 4. | Scott, B. D. (1978) Australian Journal of Marine and Freshwater Research 29, 31-44. |
| 5. | Scott, B. D. (1979) Australian Journal of Marine and Freshwater Research 30, 449-61. |
| 6. | Thompson, P. A. & Hosja, W. (1996) Marine & Freshwater Research 47, 659-667. |
| 7. | D'Adamo, N., Simpson, C., Mills, D., Imberger, J. & McComb, A. (1992) Science of the Total Environment, Supplement , 829-850. |
| 8. | May, V. (1981) Australian Journal of Ecology 6, 329-43. |
| 9. | Underwood, A. J. (1981) Journal of Marine Biology and Ecology 51, 57-85. |
| 10. | Kirkman, H. (1985) Aquatic Botany 21, 363-375. |
| 11. | Kirkman, H. (1978) Aquatic Botany 5, 63-76. |
| 12. | Kendrick, G. A., Jacoby, C. A. & Heinemann, D. (1996) Hydrobiologia 327, 283-289. |
| 13. | Neverauskas, V. P. (1987) Estuarine, Coastal and Shelf Science 25, 509-17. |
| 14. | Neverauskas, V. P. (1987) Marine Pollution Bulletin 18, 158-64. |
| 15. | Saenger, P., Stephenson, W. & Moverley, J. (1979) Memoirs of the Queensland Museum 19, 399-412. |
| 16. | Gaughan, D. J. & Potter, I. C. (1995) Estuarine, Coastal and Shelf Science 41, 117-35. |
| 17. | Geddes, M. C. (1984) Australian Journal of Marine and Freshwater Research 35, 417-26. |
| 18. | Neale, I. M. & Bayly, I. A. E. (1974) Australian Journal of Marine and Freshwater Research 25, 337-50. |
| 19. | Schlacher, T. A. & Wooldridge, T. H. (1995) Cahiers de Biologie Marine 36, 211-227. |
| 20. | Taw, N. & Ritz, D. A. (1979) Australian Journal of Marine and Freshwater Research 30, 179-202. |
| 21. | Taw, N. & Ritz, D. A. (1978) Australian Journal of Marine and Freshwater Research 29, 763-75. |
| 22. | Chalmer, P. N., Hodgkin, E. P. & Kendrick, G. W. (1976) Records of the Western Australian Museum 4, 383-409. |
| 23. | Edgar, G. J. (1990) Journal of Experimental Marine Biology and Ecology 144, 205-234. |
| 24. | Edwards, P. B. (1989) Australian Journal of Marine and Freshwater Research 40, 69-78. |
| 25. | Gibbs, P. J., Collins, A. J. & Collett, L. C. (1980) Australian Journal of Marine and Freshwater Research 31, 509-16. |
| 26. | Haynes, D. & Quinn, G. P. (1995) Australian Journal of Marine and Freshwater Research 46, 931-42. |
| 27. | Jones, A. R., Watson-Russell, C. J. & Murray, A. (1986) Australian Journal of Marine and Freshwater Research 37, 521-43. |
| 28. | Jones, A. R. (1987) Australian Journal of Marine and Freshwater Research 38, 607-24. |
| 29. | Martin-Smith, K. M. (1993) Journal of Experimental Marine Biology and Ecology 174, 243-260. |
| 30. | Morrisey, D. J., Howitt, L., Underwood, A. J. & Stark, J. S. (1992) Marine Ecology Progress Series 81, 197-204. |
| 31. | Morrisey, D. J., Underwood, A. J., Howitt, L. & Stark, J. S. (1992) Journal of Experimental Marine Biology and Ecology 164, 233-245. |
| 32. | Morrisey, D. J., Underwood, A. J. & Howitt, L. (1996) Marine Biology 125, 199-213. |
| 33. | Moverley, J., Saenger, P. & Curtis, M. A. (1986) Hydrobiologia 134, 227-35. |
| 34. | Papas, P. J. (1994) in The effect of a saline wedge on the macrobenthos of the upper Swan River estuary Murdoch University, Western Australia. |
| 35. | Poore, G. C. B. (1982) Australian Journal of Marine and Freshwater Research 33, 901-15. |
| 36. | Poore, G. C. B. & Rainer, S. (1979) Estuarine, Coastal and Shelf Science 9. |
| 37. | Poore, G. C. B. & Kudenov, J. D. (1978) Australian Journal of Marine and Freshwater Research 29, 141-55. |
| 38. | Rainer, S. F. (1982) Estuarine, Coastal and Shelf Science 15, 423-41. |
| 39. | Rainer, S. (1981) Estuarine, Coastal and Shelf Science 13, 597-620. |
| 40. | Rainer, S. F. & Fitzhardinge, R. C. (1981) Australian Journal of Marine and Freshwater Research 32, 227-43. |
| 41. | Saenger, P., Stephenson, W. & Moverley, J. (1980) Memoirs of the Queensland Museum 20, 143 -61 |
| 42. | Saenger, P., Stephenson, W. & Moverley, J. (1982) Australian Journal of Marine and Freshwater Research 33, 1083-95. |
| 43. | Gordon, D. M. & McComb, A. J. (1989) Water Research 23, 633-645. |
| 44. | Lavery, P. S., Lukatelich, R. J. & McComb, A. J. (1991) Estuarine, Coastal and Shelf Science 33, 1-22. |
| 45. | Congdon, R. A. & McComb, A. J. (1979) Aquatic Botany 6, 121-132. |
| 46. | Cambridge, M. L. & McComb, A. J. (1984) Aquatic Botany 20, 229-43. |
| 47. | Gray, J. S., Clarke, K. R., R.M., W. & Hobbs, G. (1990) Marine Ecology Progress Series 66, 285-299. |
| 48. | Ganf, G. G., Stone, S. J. L. & Oliver, R. L. (1986) Australian Journal of Marine and Freshwater Research 37, 183-97. |
| 49. | Murray, L., Dennison, W. C. & Kemp, W. M. (1992) Aquatic Botany 44, 83-100. |
| 50. | Edgar, G. J., Shaw, C., Watson, G. F. & Hammond, L. S. (1994) Journal of Experimental Marine Biology and Ecology 176, 201-26. |
| 51. | Keough, M. J. & Quinn, G. P. (1991) Australian Journal of Marine and Freshwater Research 42, 539-54. |
| 52. | Kimmerer, W. J. & McKinnon, A. D. (1985) Estuarine, Coastal and Shelf Science 21, 145-59. |
| 53. | Kimmerer, W. J. & McKinnon, A. D. (1989) Marine Ecology Progress Series 53, 21-35. |
| 54. | Schlacher, T. A. & Wooldridge, T. H. (1996) Journal of Experimental Marine Biology & Ecology 201, 159-171. |
| 55. | Chapman, M. G. (1998) Marine Ecology Progress Series 162, 71-8. |
| 56. | Chessman, B. C. & Robinson, D. P. (1987) Australian Journal of Marine and Freshwater Research 38, 289-99. |
| 57. | Cheal, F., Davis, J. A., Growns, J. E., Bradley, J. S. & Whittles, F. H. (1993) Hydrobiologia 257, 47-56. |
| 58. | Edgar, G. J. (1990) Journal of Experimental Marine Biology and Ecology 137, 215-40. |
| 59. | Geddes, M. C. (1987) Transactions of the Royal Society of South Australia 111, 173-181. |
| 60. | Hatcher, A. (1989) Australian Journal of Marine and Freshwater Research 40, 79-96. |
| 61. | Jones, G. & Candy, S. (1981) Australian Journal of Marine and Freshwater Research 32, 379-98. |
| 62. | McGuinness, K. A. (1984) Journal of Biogeography 11, 439-456. |
| 63. | John, J. (1988) in The distribution of epiphytic diatoms in the Swan River Estuary, Western Australia, in relation to hydrological factors, pp. 335-344. |
| 64. | Fairweather, P. G. (1997) in Determining the health of estuaries: Priorities for ecological research Deakin University, Warrnambool. |
| 65. | Hillman, K. (1985) in The production ecology of the seagrass Halophila ovalis (R. Br.) Hook, in the Swan/Canning estuary, Western Australia University of Western Austrailia. |
| 66. | Hutchings, P. (1992) Marine Pollution Bulletin 25, 196-99. |
| 67. | Jones, G. J., Blackburn, S. I. & Parker, N. S. (1994) Australian Journal of Marine & Freshwater Research 45, 787-800. |
| 68. | Hosja, W. & Deeley, D. M. (1993) in Harmful phytoplankton surveillance in Western Australia. Waterways Commission of Western Australia. |
| 69. | Everaarts, J. M. & Sarkar, A. (1996) Water Science and Technology 34, 157-162. |
| 70. | Laureillard, J. & Saliot, A. (1993) Marine Chemistry 43, 247-61. |
| 71. | Clarke, G. M. (1994) Australian Biologist 7, 94-104. |
| 72. | Foale, S. (1993) Marine Pollution Bulletin 26, 546-5 |
| 73. | Walker, D. I., Paling, E. I., Sim, C., Pennifold, M., Manning, C. & Kirkman, H. (1995) Water Authority of Western Australia Report E3.1 |
| 74. | Harris, G. P. (1994) LWRRDC Report 12/94 |
<TITLE>Assessing the ecological health of estuaries in Australia</TITLE>
<META NAME="DC.Title" CONTENT="Assessing the ecological health of estuaries in Australia">
<META NAME="DC.Creator" CONTENT="D.M. Deeley and E.I. Paling Marine and Freshwater Research Laboratory Institute for Environmental Science Murdoch University ">
<META NAME="DC.Type" CONTENT="text">
<META NAME="DC.Date" CONTENT="1999">
<META NAME="DC.Format" CONTENT="text/html">
<META NAME="DC.Coverage" CONTENT="Australia">
<META NAME="DC.Description" CONTENT="Australia covers a wide range of geographical and climatic regions from the northern tropics to cool southern temperate regions and the Mediterranean climate of the southwest. There is considerable variation in the pattern of rainfall for estuarine catchments throughout Australia. As a consequence of interactions between rainfall and geomorphology Australian estuaries display a wide diversity of forms across regions. The nature of impacts caused by increased sediment and pollutant loads on biological communities in estuaries throughout Australia is not well understood, even though there have been numerous investigations in some estuaries, particularly those surrounded by major cities. This lack of system-wide understanding has arisen partly because of the dynamic nature of Australian estuaries and the requirement for extensive spatial and temporal data sets to describe natural variability of physico-chemical and biological processes. The ongoing selection, evaluation and refinement of environmental indicators for assessing the ecological health of Australian estuaries, needs to proceed as a close partnership between land and waterway managers and scientific specialists. A hierarchy of environmental indicators is required for Australian estuaries, which provide for assessment of current status, a measure of diagnostic precision and a robust predictive capacity - early warning. Of the range of potential indicators evaluated in this review, some core indicators have been used successfully by managers, some will require further development and others will need considerable additional research before links between stress and response have been established.">
<META NAME="DC.Relation" CONTENT="This report describes the outcomes of the research projects conducted under the Urban Research and Development sub-program of the National River Health Program (NRHP). The NRHP is an on-going national program established in 1993, managed by the Land and Water Resources Research and Development Corporation (LWRRDC) and Environment Australia. Its mission is to improve the management of Australia's rivers and floodplains for their long-term health and ecological sustainability">
<META NAME="DC.Source" CONTENT="Environment Australia community Information Unit">
<META NAME="DC.Subject" CONTENT="Water, rivers">
<META NAME="DC.Publisher" CONTENT="Land and Water Australia (formerly Land and Water Resources Research and Development Corporation). GPO Box 2182 Canberra ACT 2601">
<META NAME="DC.Publisher" CONTENT="Published Electronically on au.riversinfo.org by the Environmental Information Association (Incorporated) with the permission of LWRRDC and Environment Australia.">
<META NAME="DC.Rights" CONTENT="Copyright (©) LWRRDC">
<META NAME="DC.Identifier" CONTENT="Assessing the ecological health of estuaries in Australia, D.M. Deeley and E.I. Paling. Marine and Freshwater Research Laboratory, Institute for Environmental Science Murdoch University. LWRRDC Occasional Paper 17/99 (Urban Subprogram, Report No. 10) December 1999 ISBN: 0 642 26767 7">
<META NAME="DC.Identifier" CONTENT="http://au.riversinfo.org/library/nrhp">
<META NAME= "DC.Language" CONTENT="en">
 Riversinfo Australia (au.riversinfo.org): Publication Information:
Riversinfo Australia (au.riversinfo.org): Publication Information:
Current at December 2002
 au.riversinfo.org has been developed to facilitate public access to scientific and technical information on Australia's Rivers. Much of this material was paid for using public funds and is therefore owned by the Australian people. Environment Australia have been instrumental in making available all the publications from:
au.riversinfo.org has been developed to facilitate public access to scientific and technical information on Australia's Rivers. Much of this material was paid for using public funds and is therefore owned by the Australian people. Environment Australia have been instrumental in making available all the publications from:
 The au.riversinfo.org Internet site is in-part supported by the Natural Heritage Trust. The Natural Heritage Trust focuses on five key environmental themes - land, vegetation, rivers, coasts and marine, and biodiversity. The programs of the Natural Heritage Trust play a major role in developing sustainable agriculture and natural resource management, as well as protecting our unique biodiversity through improved management and delivery of resources.
The au.riversinfo.org Internet site is in-part supported by the Natural Heritage Trust. The Natural Heritage Trust focuses on five key environmental themes - land, vegetation, rivers, coasts and marine, and biodiversity. The programs of the Natural Heritage Trust play a major role in developing sustainable agriculture and natural resource management, as well as protecting our unique biodiversity through improved management and delivery of resources.
 The au.riversinfo.org Internet site is in-part supported by Sydney Airport who have co-funded a Natural Heritage Trust supported project intended to develop Internet based environmental information integration and management systems. This will assist in developing an overall perspective on the demands and uses for environmental resources
The au.riversinfo.org Internet site is in-part supported by Sydney Airport who have co-funded a Natural Heritage Trust supported project intended to develop Internet based environmental information integration and management systems. This will assist in developing an overall perspective on the demands and uses for environmental resources